The International Tinnitus Journal
Official Journal of the Neurootological and Equilibriometric Society
Official Journal of the Brazil Federal District Otorhinolaryngologist Society
ISSN: 0946-5448

Google scholar citation report
Citations : 12448
The International Tinnitus Journal received 12448 citations as per google scholar report
The International Tinnitus Journal peer review process verified at publons
Indexed In
- Excerpta Medica
- Scimago
- SCOPUS
- Publons
- EMBASE
- Google Scholar
- Euro Pub
- CAS Source Index (CASSI)
- Index Medicus
- Medline
- PubMed
- UGC
- EBSCO
Volume 21, Issue 2 / December 2017
Research Paper Pages:179-184
In Vitro Differentiation of Human Bone Marrow Mesenchymal Stem Cells to Hair Cells Using Growth Factors
Authors: Mohammad-Reza Mahmoudian-Sani, Morteza Hashemzadeh-Chaleshtori, Mohammad-Saeid Jami, Massoud Saidijam
PDF
Abstract
Objective: In this study, we attempted to differentiated human bone marrow-derived mesenchymal stem cells) hBMSCs (to auditory hair cells using growth factors. Methods: Retinoic acid (RA), basic fibroblast growth factor (bFGF), and epidermal growth factor (EGF) were added to hBMSCs cell culture medium. The cells were evaluated morphologically and the expression of SOX2, POU4F3, MYO7A, and Calretinin at mRNA level and ATOH1 mRNA and protein expression. Results: After treatment with the growth factors, the morphology of the cells did not change, but evaluation of gene expression at the mRNA level increased the expression of the ATOH1, SOX2, and POU4F3 markers. Growth factors increased the expression of ATOH1 at the protein level. The expression of calretinin showed decreased and MYO7A no significant change in expression. Conclusion: hBMSCs have the potential to differentiate to hair cell-like using the RA, bFGF, and EGF.
Keywords: bone marrow mesenchymal stem cells, hair cell, growth factor
Introduction
The sensorineural hearing loss is one of the most common disabilities worldwide [1] . It is often believed that deafness occurs in humans due to the loss or impairment of the function of the hair cells in the cochlea of the ear, when hair cells cannot be regenerated spontaneously; in this case, the treatment of patients with severe to deep deafness is comparatively more difficult [2] . Nowadays, cochlear implants are the only way to restore auditory function in patients with deep deafness. However, despite the best results, normal hearing will not be fully restored [3] . Therefore, an alternative method is needed to treat profound deafness. Deafness occurs in humans when the hair cells are damaged due to disease or trauma, noise or age. In fish amphibians, and birds but not mammals, it is possible to replace damaged hair cells [4,5] . Owing to their importance in the functioning of the inner ear, hair cells have attracted more attention. Hair cells are essential for converting sound stimuli to neural signals. The hair cells are the main components of the inner ear that facilitates mechanoelectrical transmission to understand the sound [6] . These cells are highly differentiated and have many special characteristics, which make it difficult to regenerate them. Practical methods have not yet been offered for the regeneration of the hair cells. In early 2000, cell transplantation, techniques focused on the reconstruction of the hair cells. However, the migration of the transplanted stem cells to the damaged sensory epithelium of the inner ear occurs rarely [7] .
Stem cell therapy
Although it is important to study embryonic stem cells, there are serious concerns about malignancies associated with these cells. Generation of induced pluripotent stem cell (iPSC) from adult or somatic cells is associated with gentical changes. Genomic changes are a risk factor for mutagenesis and the development of malignant cell transformation [8] . Genetic defects in adult or somatic cell-derived iPSCs not only cause tumorigenicity, but also lead to impairment of differentiation. Further research is required for the clinical use of these cells [9] . Mesenchymal stem cells are the first candidate for cell therapy, because these cells are located at the target organ, and therefore, have been potential to replace the damaged cells. In addition to the ability to differentiate to functional cell types, these cells release a wide spectrum of differentiating growth factors, cytokines, chemokines, microvesicles or exosomes that facilitated the lateral transmission of organ-protecting signals, and have potent anti-inflammatory immunomodulatory properties to improve the proliferation of endogenous stem cells [10] . They are consequently used to repair the tissues.
BMSCs in the treatment of sensorineural hearing loss
BMSCs have been used in a variety of regenerative medical fields [11] . The use of BMSCs in the inner ear has been studied (Table 1). The fate of autologous BMSCs after intra cochlear transplantation was also investigated. Autologous BMSCs in the cochlea were observed to differentiate to neurons [12] . In addition, the transplanted BMSCs migrate to different parts of the cochlea. Consistent findings have been reported regarding the transplantation of the BMSCs [13] . The transplanted BMSCs migrated widely into the spiral ligament, which was used to regenerate the specific fibrocytes in the spiral ligament. These findings suggest that BMSCs can be used for the treatment of sensorineural hearing loss due to the loss of ligand-specific fibrocytes [14] . When the source of the cell does not exist in the inner ear, induction of differentiation, for example, induction of differentiation from stem cells to cochlear hair cells is a useful method to restore hair cells through regeneration (Table 1).
| Study Model | Main results and mechanisms of action of BM-MSCs in hearing loss repair | Ref |
|---|---|---|
| Guinea pig | Results show the potential of NI-hBM-MSCs to give rise to replace the lost cochlear cells in hearing loss mammals. | 15 |
| Rat | Migration of MSCs into the cochlea was accompanied by the expression of BDNF, systemic delivery of BM-MSCs may be good option for the stem cell treatment of hearing loss. | 16 |
| Mice | MSCs engraftment to the cochlea and differentiated into type II and V fibrocyte-like cells, BM-MSCs successfully transplanted into the cochlea ofmice and differentiated into fibrocyte-like. | 17 |
| Guinea pig | BDNF gene modified BM- MSC could maintain expression for at least 28 days after transplantation into cochlea of drug deafened guinea pigs. | 18 |
| Rat | TransplantedBM-MSCs actually invaded the injured area and contributed to the structural reorganization of the injured cochlea, BM-MSCs transplantation improves incomplete hearing recovery. | 14 |
| Mice | Demonstrated the homing capability of BM-MSCs to the deafened cochlea, and these cells displayed mature hematopoietic properties, upregulation of SDF-1 expression, mobilize, migrateMSCsto the site of injury. | 19 |
| Mongolian gerbil | Survival of transplanted MSCs into the modiolus of the cochlea may result in regeneration of damaged SGNs, undifferentiatedBM-MSCswere able to survive in the modiolus both in the control cochlea and the ouabain-treated cochlea. | 20 |
| Guinea pig | ABR results showed mild hearing recovery after transplantation. neural differentiated BM-MSCs are able to restore damaged SGNs and decrease hearing thresholds in an AN model. | 21 |
Table 1. Studies of BMSCs-based therapies for treatment of hearing loss [15-21].
Transdifferentiation for hair cell regeneration
Transdifferentiation is defined as the conversion of one differentiated cell type into another [22] . Transdifferentiation may be achieved in several ways by using extracellular growth factors, individual transcription factors, or combinations of the two. Research showed that BMSCs differentiate into mesodermal cell types and also reprogram to transdifferentiate into endodermal and ectodermal cell types [23] . Transdifferentiation is a useful method to regenerate hair cells. However, the method that have so far been reported only to manipulate one factor, and hair cell restoration is also ineffective. Therefore, an examination of the more effective combination of regenerative agents is needed to achieve the regeneration of the hair cell for clinical uses [24] . Recently, quantitative factors have been manipulated to induce transdifferentiation of the cochlear hair cells, and the induced hair cells are not exactly similar to physiological cells. This indicates that a single factor is not sufficient and several factors should be used for regenerating hair cells. It is important for use of re-programming factors to achieve complete regeneration of the hair cells. Using growth factors and the ATOH1 transcription factor, miRNAs and inhibiting the Notch signal is good option to achieve this goal. The use of growth factors to differentiate stem cells to the hair cells is important. In natural development, the signaling of growth factors is very essential to the development of the inner ear [25] .
Materials and Methods
Human BMSCs were purchased from the bank of BMSCs of the Cell Therapy Center of the Royan Institute (Tehran, Iran) and cultured in the Dulbecco's Modified Eagle's (DMEM) medium containing 15% fetal bovine serum (FBS) and 1% streptomycin and penicillin. The medium was replaced every three days, and the third passage were used to differentiate into hair cells. To differentiate hBMSCs to hair cell precursors (Neurosphere), hBMSCs were first cultured on medium DMEM/F12 containing 20 ng/ml of epidermal growth factor (EGF) (Invitrogen) and 10 ng/ml of basic fibroblast growth factor (bFGF) (Invitrogen) for four days. Then, for differentiating to hair cell the neurospheres grown in DMEM/F12 medium containing EGF and retinoic acid (RA) for 12 days (Table 2). The cells were kept for 12 days on a medium containing 20 ng/ml of EGF and 1 μM of RA in serum-free DMEM-F12. The culture medium was replaced every four days.
| Neurosphere medium | day | HCmedium | day |
|---|---|---|---|
| DMEM: F12 | DMEM: F12 | ||
| bFGF 20 ng/mL | 4 | 20 ng/ml EGF | 12 |
| EGF 10 ng/mL | 1 µM RA | ||
| 1% Antibiotic–antimycotic | 1% Antibiotic–antimycotic |
Table 2. Media for inducing differentiation into neurospheres and hair cells.
Real-time qRT-PCR to evaluate the expression of genes
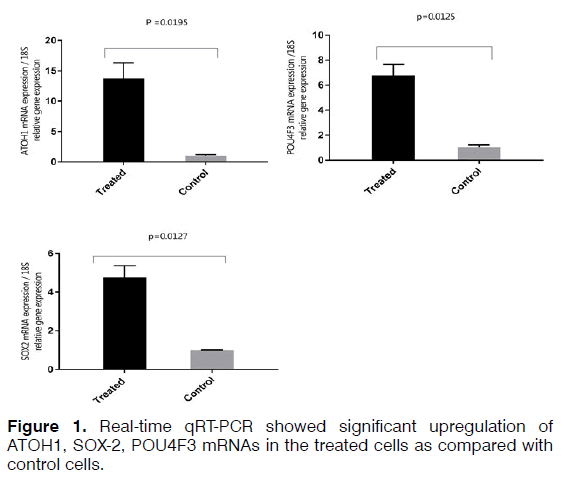
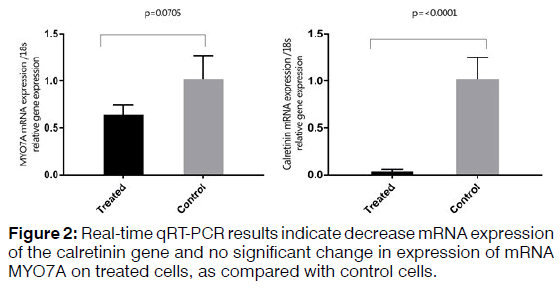
To isolate total RNA from the cells, TRIzol Reagent (Ambion) was used based on the manufacturer's protocol. RNA samples were measured using a spectrophotometer (NanoDrop®, Thermo Fisher Scientific) and evaluated qualitatively and quantitatively. Two microgram of total RNA used to synthesize cDNA using a kit (Thermo scientific-K1622). The thermal conditions consisted of a denaturation of 3 min at 95°C followed by 40 cycles at 95°C for 15s and 72°C for 25s. The primers used for each of the studied genes are shown in Table 3. Relative quantities were calculated using the 2^(ΔΔCt) method. 18S used as internal controls for mRNA quantification. A t-test was used to investigate the difference in the expression of mRNA between the treated and control samples in the Real-time qRT-PCR method. The difference between control and treated groups was considered statistically significant if p < 0.05 (Figures 1 and 2).
| Name | Sequence |
|---|---|
| POU4F3 forward | 5′- AGTCTCTCACTCTCTCGCACAA- 3′ |
| POU4F3 reverse | 5′- GCTGTTCTTCTCTCGGTAGGC- 3′ |
| MYO7A forward | 5′- GTGGTGAAGCTCTGCGACT- 3′ |
| MYO7Areverse | 5′- GCATAGGCTTGATGTGCGTT- 3′ |
| Calretinin forward | 5′- CGCAGACGGAAATGGGTATATTG- 3′ |
| Calretininreverse | 5′- ATCATACTTCTGCATGAACTCCTT- 3′ |
| ATOH1 forward | 5′- CCCCTTCCAGCAAACAGGTG- 3′ |
| ATOH1 reverse | 5′- ACGGGATAACATTGCGCAGC- 3′ |
| SOX2 forward | 5′- GGGGAAAGTAGTTTGCTGCC- 3′ |
| SOX2 reverse | 5′- CGCCGCCGATGATTGTTATT- 3′ |
| 18S forward | 5′- GTAACCCGTTGAACCCCATTCGT- 3′ |
| 18S reverse | 5′- ACCATCCAATCGGTAGTAGCGACG- 3′ |
Table 3. Primers used in Real-time qRT-PCR for mRNAs.
Western blotting
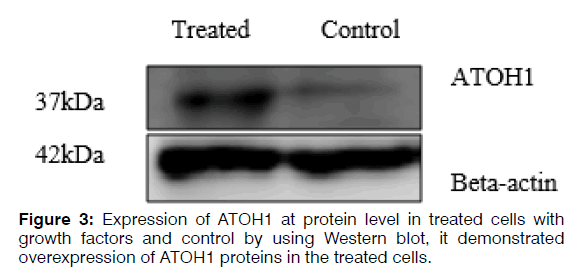
Total cell protein was extracted using urea lysis buffer (8 M) and approximately 30 μg of total protein were fractionated by 12% SDS-PAGE and transferred to a PVDF membrane. After the transfer, the PVDF membrane was blocked in 3% BSA in TBST at room temperature for 1 hr and then incubated overnight in primary antibody ATOH1 (ab137534) at 1/2000 dilution at 4°C, then incubated in the HRP-conjugated secondary antibody (ab97051) solution for 1 hr at room temperature. The membrane was then incubated with antibody against beta-actin (4970s) to ensure the same loading of proteins in the gel. Finally, bands were visualized using the ECL kit according to the manufacturer’s instructions (Figure 3).
Results
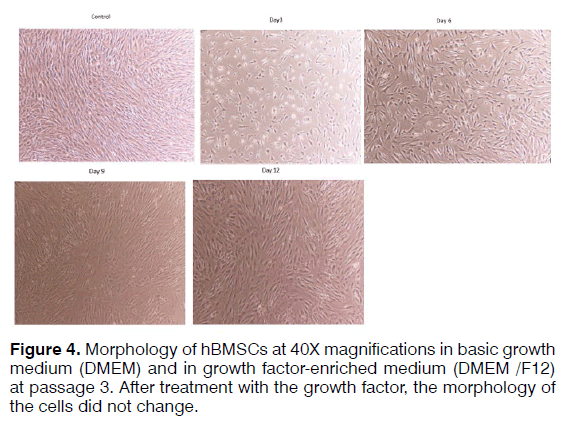
Morphological features of hBMSCs cells at passage 3 (P3) grown using two different media were morphologically compared (Figure 4).
Discussion
The differentiation of BMSCs to inner ear hair cells in vitro is considered a milestone to understand the regeneration of inner ear hair cells based on stem cells. The induction of hair cell from stem cells will gradually become a reliable method. This method will be used to explain the mechanism of inner ear diseases or to screen for the drug. It is necessary to improve induction, and to provide a method that specifically induces hair cells. There are many problems with the use of in vivo induced hair cells. It is impossible to transplant adult stem cells and fit them well into the organ of Cori, and other approaches are needed [26] . Growth factors are crucial factors in the development of the ear. EGF plays a part in the inner ear. EGF stimulates cell proliferation in the atrial sensory epithelium. In a study, human embryonic stem cells were cultured as a single layer and synergistically treated with FGF3 and FGF10 to induce the fate of the otic placode in mice. Epithelial cell-like colonies have been reported to differentiate more in an RA- or EGF-enriched medium [27] . The proliferation and regeneration of stem/ stem-like cells in the adult sensory epithelium require the simultaneous release mitogenic EGF or the insulin-like growth factor1 [28,29] . RA is a morphogenic agent who acts on the anterior-posterior axis of the ear [30] . FGFs also are key factors for the development of the organ of Corti. In mice, signaling of fibroblastic growth factors (FGFs) is involved in the early events of induction of auditory vesicles. Specific deletion of FGFR1 results in severe impairment of the development of hair cells and precursor cells [31] . FGF20 is specifically needed to differentiate the side constituents of the cochlea (including outer hair cells and Dieter's cells) [32] . In patients with low-frequency hearing, who undergo cochlear implantation, the maintenance of the remaining hair cells is essential. Growth factors can prevent hair cell damage or even regenerate hair cells [33] . The RA pathway is activated immediately after the loss of hair cells, accelerating the proliferation of cell precursors, and regulating the expression of two key genes, P27kip and SOX2. The results have indicated that RA can be used as an essential signal for the regeneration of hair cells [34] . In the cochlea of rats fed with all-trans retinoic acid (ATRA), the protection of the organ of corti is more likely to occur than in the cochlea of those feds with oil and saline. ATRA inhibits JNK activation. The results have shown that ATRA has an anti-apoptotic effect on the noise-exposed cochlea [35] . ATRA increases the expression of Prdx6 and improves hearing loss in noise-induced hearing loss (NIHS). Prdx6 induction may be associated with the rapid treatment of NIHS [36] . Inhibition of the RA signaling causes a significant reduction on the number of hair cells. The cells in the developing cochlea respond to RA, and RA signals are critical to the normal growth of the organ of corti. After exposure to noise, ATRA can help preserve hearing. Also, when acoustic trauma occurs accidentally, RA has a protective effect. RA is a clinical drug that is used orally and considered to be healthy. Although the findings can also be used for human, further research is needed to determine the safe and effective dose, duration and time of initiation of drug administration for practical application [37] . The first known gene whose expression is associated with hair cell is a basic helix-loop-helix (bHLH) transcription factor called ATOH1 [38] . During the development process, the expression of ATOH1 is limited to cells that ultimately differentiate into hair cells. The expression of ATOH1 in the bird's cochlea increases during the process of regeneration of the hair cells [39] . Among the several transcription factors associated to the evolution of hair cells, ATOH1 is the most promising factor for the regeneration of hair cells. Purposeful loss of ATOH1 leads to complete destruction of the hair cells [40] . In order to regenerate hair cell, transport of a specific gene associated with induction of a hair cell, such as ATOH1, was reported to be effective in mouse [41] . An increase in the expression of ATOH1 in the guinea-pig cochlea after drug-induced hair cell damage led to the retrieval of the hair cell function, in addition to repairing the hair cell [41] . ATOH1 is a major gene to commit to the fate of the hair cells, but we still do not know how the expression of ATOH1 is induced in transplanted cells in vivo, as well as what other genes are needed for the differentiation of the hair cells.
Conclusion
Regenerating hair cells with physiological function is considered a challenge for researchers. Using different types of growth factors that are known for regenerating hair cells increases the possibility of obtaining hair cells phenotype. Real-time qRT-PCR analysis demonstrated increased expression of SOX2, which is a critical gene in the development of cochlear hair cells, the receptor cells for hearing. SOX2 is expressed in the neurosensory domain of the otic placode. The real-time qRT-PCR analysis also revealed increased expression of POU4F3, which is a transcription factor known to play an essential role for the survival of hair cells. ATOH1 is a major gene for committing the fate of the hair cell. Analysis of gene and protein expression in our study showed that the expression of ATOH1 increased in the hBMSCs treated with the RA, bFGF, and EGF. However, the expression of calretinin decreased and no significant change in expression levels of mRNA MYO7A. The hBMSCs, which the expression of the ATOH1, SOX2, and POU4F3 marker's increase, can get a new approach to regenerate inner ear sensory hair cells. In this study, we showed that hBMSCs have the potential to differentiate in auditory hair cell-like. Current findings point about the importance to the application of BMSCs in the regenerative medicine for the inner ear. Recent developments in biology and rapid advances in the field of stem cell biology are expected to lead to the resolution of problems that prevent successful mammalian hair cell regeneration.
Acknowledgements
This work was supported by Research Deputy of Hamadan University of Medical Sciences (grant number 9409245160) and was a part of Ph.D. Thesis of Mr. Mahmoudian Sani.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Mehri-Ghahfarrokhi A, Hashemzadeh-Chaleshtori M, Shojaeian A, Mahmoudian-Sani M. Studying gap junction beta 2-related deafness in iranian population. Otorinolaringologia. 2017;67(3):89-95.
- Wong ACY, Ryan AF. Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front Aging Neurosci. 2015;7:58.
- Mahmoudian-sani MR, Mehri-Ghahfarrokhi A, Hashemzadeh-Chaleshtori M, Saidijam M, Jami MS. Comparison of three types of mesenchymal stem cells (Bone marrow, adipose tissue, and umbilical cord-derived) as potential sources for inner ear regeneration. Int Tinnitus J. 2017;21(2):121-6.
- Mangiardi DA, McLaughlin-Williamson K, May KE, Messana EP, Mountain DC, Cotanche DA. Progression of hair cell ejection and molecular markers of apoptosis in the avian cochlea following gentamicin treatment. J Comp Neurol. 2004;475(1):1-18.
- Stone JS, Cotanche DA. Identification of the timing of S phase and the patterns of cell proliferation during hair cell regeneration in the chick cochlea. J Comp Neurol. 1994;341(1):50-67.
- Fettiplace R. Hair cell transduction, tuning and synaptic transmission in the mammalian cochlea. Comp Physiol. 2017;7(4):1197-227.
- Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Geleoc GS, et al. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007;8(1):18-31.
- Romano G, Morales F, Marino IR, Giordano A. A commentary on iPS cells: Potential applications in autologous transplantation, study of illnesses and drug screening. J Cell Physiol. 2014;229(2):148-52.
- Koyanagi-Aoi M, Ohnuki M, Takahashi K, Okita K, Noma H, Sawamura Y, et al. Differentiation-defective phenotypes revealed by large-scale analyses of human pluripotent stem cells. Proc Natl Acad Sci USA. 2013;110(51):20569-74.
- Kim HJ, Park J-S. Usage of human mesenchymal stem cells in cell-based therapy: Advantages and disadvantages. Dev Reprod. 2017;21(1):1-10.
- Mahla RS. Stem cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol. 2016;2016:6940283.
- Naito Y, Nakamura T, Nakagawa T, Iguchi F, Endo T, Fujino K, et al. Transplantation of bone marrow stromal cells into the cochlea of chinchillas. Neuroreport. 2004;15(1):1-4.
- Sharif S, Nakagawa T, Ohno T, Matsumoto M, Kita T, Riazuddin S, et al. The potential use of bone marrow stromal cells for cochlear cell therapy. Neuroreport. 2007;18(4):351-4.
- Kamiya K, Fujinami Y, Hoya N, Okamoto Y, Kouike H, Komatsuzaki R, et al. Mesenchymal stem cell transplantation accelerates hearing recovery through the repair of injured cochlear fibrocytes. Am J Pathol. 2007;171(1):214-26.
- Jang S, Cho HH, Kim SH, Lee KH, Jun JY, Park JS, et al. Neural-induced human mesenchymal stem cells promote cochlear cell regeneration in deaf guinea pigs. Clin Exp Otorhinolaryngol. 2015;8(2):83-91.
- Choi BY, Song JJ, Chang SO, Kim SU, Oh SH. Intravenous administration of human mesenchymal stem cells after noise- or drug-induced hearing loss in rats. Acta oto-laryngologica. 2012;132(Suppl 1):S94-102.
- Kasagi H, Kuhara T, Okada H, Sueyoshi N, Kurihara H. Mesenchymal stem cell transplantation to the mouse cochlea as a treatment for childhood sensorineural hearing loss. Int J Pediatr Otorhinolaryngol. 2013;77(6):936-42.
- Chen GG, Xie DH, Liu QX, Tan ZQ. [Expression of brain-derived neurotrophic factor modified bone marrow mesenchymal stem cells in the cochlea of drug deafened guinea pigs and its protection role]. Zhonghua er bi yan hou tou jing wai ke za zhi = Chinese journal of otorhinolaryngology head and neck surgery. 2010;45(11):924-9.
- Tan BT, Lee MM, Ruan R. Bone-marrow-derived cells that home to acoustic deafened cochlea preserved their hematopoietic identity. J CompNeurol. 2008;509(2):167-79.
- Matsuoka AJ, Kondo T, Miyamoto RT, Hashino E. Enhanced survival of bone-marrow-derived pluripotent stem cells in an animal model of auditory neuropathy. Laryngoscope. 2007;117(9):1629-35.
- Cho YB, Cho HH, Jang S, Jeong HS, Park JS. Transplantation of neural differentiated human mesenchymal stem cells into the cochlea of an auditory-neuropathy guinea pig model. J Korean Med Sci. 2011;26(4):492-8.
- Tosh D, Slack JM. How cells change their phenotype. Nat Rev Mol Cell Biol. 2002;3(3):187-94.
- Otify DY, Youssef E, Nagy NB, Marei MK, Youssif MI. Transdifferentiation of Bone marrow mesenchymal stem cells into neural cells via cerebrospinal fluid. Biomed Biotechnol. 2014;2(4):66-79.
- Atkinson PJ, Huarcaya Najarro E, Sayyid ZN, Cheng AG. Sensory hair cell development and regeneration: Similarities and differences. Development. 2015;142(9):1561-71.
- Okano T, Xuan S, Kelley MW. Insulin-like growth factor signaling regulates the timing of sensory cell differentiation in the mouse cochlea. J Neurosci. 2011;31(49):18104-18.
- Okano T, Kelley MW. Stem cell therapy for the inner ear: Recent advances and future directions. Trends Amplif. 2012;16(1):4-18.
- Yamashita H, Oesterle EC. Induction of cell proliferation in mammalian inner-ear sensory epithelia by transforming growth factor alpha and epidermal growth factor. Proc Natl Acad Sci USA. 1995;92(8):3152-5.
- Oesterle EC, Hume CR. Growth factor regulation of the cell cycle in developing and mature inner ear sensory epithelia. J Neurocytol. 1999;28(10-11):877-87.
- Oesterle EC, Tsue TT, Rubel EW. Induction of cell proliferation in avian inner ear sensory epithelia by insulin-like growth factor-I and insulin. J Comparative Neurol. 1997;380(2):262-74.
- Bok J, Raft S, Kong KA, Koo SK, Drager UC, Wu DK. Transient retinoic acid signaling confers anterior-posterior polarity to the inner ear. Proc Natl Acad Sci USA. 2011;108(1):161-6.
- Pirvola U, Ylikoski J, Trokovic R, Hebert JM, McConnell SK, Partanen J. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35(4):671-80.
- Huh SH, Jones J, Warchol ME, Ornitz DM. Differentiation of the lateral compartment of the cochlea requires a temporally restricted FGF20 signal. PLoS Biol. 2012;10(1):e1001231.
- Malgrange B, Rigo JM, Coucke P, Thiry M, Hans G, Nguyen L, et al. Identification of factors that maintain mammalian outer hair cells in adult organ of Corti explants. HearRes. 2002;170(1-2):48-58.
- Rubbini D, Robert-Moreno A, Hoijman E, Alsina B. Retinoic acid signaling mediates hair cell regeneration by repressing p27kip and sox2 in supporting cells. J Neurosci. 2015;35(47):15752-66.
- Ahn JH, Kang HH, Kim YJ, Chung JW. Anti-apoptotic role of retinoic acid in the inner ear of noise-exposed mice. Biochem Biophys Res Commun. 2005;335(2):485-90.
- Ahn JH, Shin JE, Chung BY, Lee HM, Kang HH, Chung JW, et al. Involvement of retinoic acid-induced peroxiredoxin 6 expression in recovery of noise-induced temporary hearing threshold shifts. Environ Toxicol Pharmacol. 2013;36(2):463-71.
- Shim HJ, Kang HH, Ahn JH, Chung JW. Retinoic acid applied after noise exposure can recover the noise-induced hearing loss in mice. Acta oto-laryngologica. 2009;129(3):233-8.
- Kelley MW. Cellular commitment and differentiation in the organ of Corti. Int J Dev Biol. 2007;51(6-7):571-83.
- Cafaro J, Lee GS, Stone JS. Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Dev Dyn. 2007;236(1):156-70.
- Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. NatNeurosci. 2004;7(12):1310-8.
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11(3):271-6.
References
1Department of Genetic & Molecular Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
2Cellular and Molecular Research Center, Basic Health Sciences Institute, Shahrekord University of Medical Sciences, Shahrekord, Iran
3Research Center for Molecular Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
Send correspondence to:
Massoud Saidijam
Department of Genetic & Molecular Medicine, Hamadan University of Medical Sciences, Hamadan, Iran. E-mail: sjam110@yahoo.com
Paper submitted to the ITJ-EM (Editorial Manager System) on December 19, 2017; and accepted on December 26, 2017.
Citation: Sani MRM, Chaleshtori MH, Jami MS, Saidijam M. In Vitro Differentiation of Human Bone Marrow Mesenchymal Stem Cells to Hair Cells Using Growth Factors. Int Tinnitus J. 2017; 21(2): 179-184