Published online Dec 27, 2014. doi: 10.4254/wjh.v6.i12.844
Revised: October 7, 2014
Accepted: October 28, 2014
Published online: December 27, 2014
Hepatocellular carcinoma (HCC) is one of the most common malignant diseases worldwide. While curative therapies, including resection, liver transplantation, and percutaneous ablation (percutaneous ethanol injection and radiofrequency ablation), are applicable for only a portion of the HCC population, transcatheter arterial chemoembolization (TACE) has been recognized as an effective palliative treatment option for patients with advanced HCC. TACE is also used even for single HCCs in which it is difficult to perform surgical resection or locoregional treatment due to systemic co-morbidities or anatomical problems. TACE has become widely adopted in the treatment of HCC. By using computed tomography-angiography, TACE is capable of performing diagnosis and treatment at the same time. Furthermore, TACE plays an important role in the multidisciplinary treatment for HCC when combined with other treatment. In this review, we first discuss the history of TACE, and then review the previous findings about techniques of achieving a locoregional treatment effect (liver infarction treatment, e.g., ultra-selective TACE, balloon-occluded TACE), and the use of TACE as a drug delivery system for anti-cancer agents (palliative, e.g., platinum complex agents, drug-eluting beads) for multiple lesions.
Core tip: Transcatheter arterial chemoembolization (TACE) has become widely adopted in the treatment of hepatocellular carcinoma (HCC). By using computed tomography-angiography, TACE is capable of performing diagnosis and treatment at the same time. Furthermore, TACE plays an important role in the multidisciplinary treatment for HCC when combined with other treatment. In this review, we first discuss the history of TACE, and then review the previous findings about techniques of achieving a locoregional treatment effect (liver infarction treatment, e.g., ultra-selective TACE, balloon-occluded TACE), and the use of TACE as a drug delivery system for anti-cancer agents (palliative, e.g., platinum complex agents, drug-eluting beads) for multiple lesions.
- Citation: Imai N, Ishigami M, Ishizu Y, Kuzuya T, Honda T, Hayashi K, Hirooka Y, Goto H. Transarterial chemoembolization for hepatocellular carcinoma: A review of techniques. World J Hepatol 2014; 6(12): 844-850
- URL: https://www.wjgnet.com/1948-5182/full/v6/i12/844.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i12.844
Hepatocellular carcinoma (HCC) accounts for one-third of cancer-related deaths worldwide, and has become the fourth leading cause of cancer death in Japan and the seventh leading cause of cancer death in the United States. In recent years, liver cancer deaths have decreased due to remarkable progress in the treatment of viral hepatitis in Japan, while HCC deaths remain high in the United States[1].
Underlying liver disease is present in most HCC cases. Development of HCC in a healthy liver is rare; the majority of patients who develop HCC have a background of chronic hepatitis/cirrhosis viral hepatitis, alcohol abuse, and/or non-alcoholic steatohepatitis. HCC frequently recurs after primary treatment due to the underlying liver disease[2,3].
With advances in diagnostic imaging and treatment in recent years, adaptation of radical treatment strategies such as surgical resection and radiofrequency ablation therapy is increasing. However, even in cases in whom curative treatment is selected as initial treatment, a high recurrence rate due to multi-centric carcinogenesis and intrahepatic metastasis makes it difficult for cure to truly be achieved. Transarterial chemoembolization (TACE) has been widely performed as a treatment for multifocal HCC in patients in whom curative treatment is difficult to perform[4-11].
In the Barcelona Clinic Liver Cancer staging system, TACE is indicated for patients with intermediate-stage HCC (four or more tumors), and in the 2010 Japan Society of Hepatology consensus-based treatment algorithm for HCC, TACE is recommended for patients with a Child-Pugh score A or B, tumor diameter of more than 3 cm, or four or more tumors. However, in real clinical conditions, TACE is selected even for single HCCs in which it is difficult to perform surgical resection or locoregional treatment due to systemic co-morbidities or anatomical problems[12,13].
TACE has been widely adopted in the treatment of HCC. Through the use of computed tomography (CT)-angiography, diagnosis and TACE can be performed at the same time. TACE also plays an important role in the multidisciplinary treatment of HCC as it is often combined with other treatments (e.g., with radiofrequency ablation, with percutaneous ethanol injection, with radiation therapy).
In this review, we discuss the history of TACE and review previous findings about techniques for achieving a locoregional treatment effect (liver infarction treatment) and the use of TACE as a drug delivery system for anti-cancer agents (palliative) for multiple lesions.
TACE induces tumor necrosis through “starvation tactics”. It takes advantage of the fact that advanced HCCs are fed only by the hepatic artery and is intended to embolize the distal portion of the hepatic artery. The liver receives blood from the portal vein and hepatic artery at a ratio of 3:1 in the normal liver. Although this ratio varies in cirrhosis, the cirrhotic liver still receives blood flow from both of these vessels. In contrast, classical HCC (moderately-differentiated type) tumors receive nutritional blood flow through the hepatic artery only, and do not depend on portal vein blood flow. By utilizing this property of HCC, TACE was developed by Yamada et al[4]. TACE has become widely used for the treatment of HCC since the 1980s. Embolization using adriamycin or mitomycin C and gelatin sponges has been carried out since the first half of the 1980s. Intraarterial injection of lipiodol with anti-cancer drugs before embolic agent results in enhanced embolic effects[14,15]. It also became apparent that using a water-in-oil type emulsion is highly effective for embolization and tumor uptake, and this method is also widely used[16].
Microcatheter insertion into the first three to four branches of the hepatic artery became easily available starting around 1990. Through the use of microcatheter injection of lipiodol into the peripheral branches of the hepatic artery, segmental TACE/subsegmental TACE became a standard treatment. Segmental TACE/subsegmental TACE allows for strong locoregional embolization while stopping the portal blood flow, thereby improving the local treatment effects of TACE[17,18]. In the 2000s, platinum complex agents became available, and treatment effects could be obtained even in HCCs that developed TACE resistance through repeat TACE[19]. Cone beam CT and flat-panel detectors have advanced imaging as they enable more accurate TACE treatment[20,21].
Intra-tumor concentrations of drugs (particularly polymer drugs) are much higher than those of normal tissue and blood due to the characteristics of blood vessels in solid tumors[14]. In hypervascular HCC, blood returns to the sinusoidal or portal vein; in addition, HCC tissue does not have associated lymph vessels. These features allow stasis of viscous liquid such as lipiodol in the sinusoidal or portal vein in or around HCCs. Nakamura et al[22] reported that liver necrosis occurs following injection of lipiodol into the hepatic artery until it is visualized in the portal vein branch. Based on this discovery, Uchida et al[15] and Matsui et al[18] developed segmental and subsegmental TACE[15,18,22].
The use of water-soluble anti-cancer drugs along with lipiodol as water-in-oil type therapy has been reported to be good for distribution of anti-cancer drugs in HCC[16,23]. Therapy involving selective infusion into tumor vessels of an anti-cancer drug/lipiodol mixture and an embolic agent (gelatin sponge) is generally called conventional TACE (cTACE), and it is widely used as standard treatment worldwide (Table 1).
| Ref. | Year | Analysis | No. of patients | Objective response (%) | Overall survival (%) | |||
| 1 yr | 2 yr | 3 yr | ||||||
| cTACE | ||||||||
| Llovet et al[7] | 2002 | Prospective | 40 | 35 (at 6 mo) | 82 | 63 | 29 | |
| Lo et al[8] | 2002 | Prospective | 40 | 39 (at 3 mo) | 57 | 31 | 26 | |
| Takayasu et al[10] | 2006 | Prospective | 8510 | NA | 82 | 63 | 47 | |
| DEB-TACE | ||||||||
| Lammer et al[52] | 2010 | Prospective | 102 | 51.6 (at 6 mo) | NA | NA | NA | |
| Sacco et al[54] | 2011 | Prospective | 33 | 100 (at 1 mo) | NA | 86.8 | NA | |
| Song et al[57] | 2012 | Retrospective | 60 | 81.6 (at 3 mo) | 88 | NA | NA | |
| Wiggermann et al[58] | 2011 | Retrospective | 22 | 22.7 (at 8 mo) | 70 | NA | NA | |
In 1983 Yamada et al[4] reported a 1-year survival rate of 44% for TACE. The 3-year survival rate of segmental TACE reported by Uchida et al[15] in 1990 was 67%, Matsui et al[18] reported a 4-year survival rate of 67% in 1993, and Takayasu et al[24] reported a 3-year survival rate of 77% using interventional radiology (IVR)-CT in subsegmental TACE in 2001. Thus, therapeutic outcomes of TACE have improved rapidly along with advances in TACE techniques, drugs, microcatheters, and the adaption of IVR-CT[25].
It has recently become possible to insert microcatheters into the distal hepatic artery more safely due to progress in microcatheter and guidewire technology. Ultra-selective TACE aims to achieve a local therapeutic effect through liver infarction. This technique involves insertion of a microcatheter selectively into a peripheral rather than subsegmental branch (subsubsegment artery), thereby wedging the tumor-feeding vessels, and then injecting lipiodol under high pressure into the tumor and surrounding sinusoids.
The local recurrence rate of ultra-selective TACE has been reported to be 7.9% at 12 mo and 17.7% at 24 mo[26]. Ultra-selective TACE allows injection of lipiodol even into hypovascular lesions in well-differentiated HCC, and is reported to have a local control rate of 53.2% in such cases[27].
With respect to pathological background, in a study of patients who underwent liver resection after undergoing ultra-selective TACE through peripheral branches, necrosis of the tumor as well as the surrounding liver parenchyma was observed[28]. With the spread of fine microcatheters as a treatment aimed at liver infarction, injection of lipiodol in the peripheral rather than the subsegmental branches is becoming a standard treatment[29].
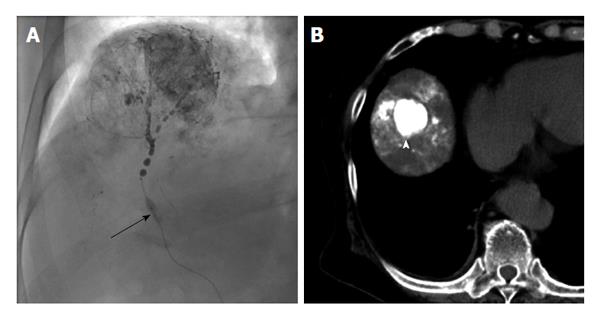
Irie et al[30] reported in 2008 that better lipiodol deposition was obtained by performing selective TACE while preventing the backflow of embolic material proximally using a micro-balloon catheter, called balloon-occluded TACE (B-TACE)[30]. In conventional TACE, lipiodol suspended with an anti-cancer drug is present in the bloodstream. The blood flow may slow before sufficient lipiodol and drug have reached the tumor, and may even stop flowing. This may occur because when the arterial blood flow is reduced, the backflow of blood to the tumor occurs from the sinusoidal and portal veins. In addition, lipiodol inflow restriction to the normal liver parenchyma is caused by a reduction in peripheral arterial pressure.
In B-TACE, hemodynamic changes caused by balloon occlusion reduce the arterial blood flow by closing the hepatic artery, thereby pushing lipiodol into the tumor under high pressure and enabling the drug to be intensively administered to the tumor, allowing for an enhanced therapeutic effect. In recent years, since micro-balloon catheters with small diameters have become more available, B-TACE has been widely used, primarily in Japan (Figure 1). In performing B-TACE, evaluation of the collateral circulation of the tumor is essential, as a good response rate is obtained if the catheter tip pressure is equal to 64 mmHg or less; the collateral circulation pressure increases above that in many cases[31].
In advanced HCC treatment, it is critical that TACE functions as an efficient drug delivery system. Platinum complex agents are anti-cancer agents that cause DNA damage. Unlike anthracyclines, which are excreted from the bile, platinum complex agents are not metabolized by P450, and are excreted primarily in the urine. Thus, platinum complex agents are considered to be advantageous for patients with liver cirrhosis. Cisplatin, a first-generation platinum complex, and miriplatin, a third-generation platinum complex, are both used in the treatment of HCC[32-39].
Kawamura et al[19] reported that a 19.6% response rate was obtained by switching the anti-cancer agent used in TACE to a platinum complex agent in cases in which the tumor number or size increased despite administration of more than one TACE treatment. Use of a platinum complex agent also resulted in a survival benefit in responders[19]. Furthermore, Maeda et al[40] also reported the efficacy of TACE using cisplatin in patients with HCC that had not responded to TACE using epirubicin, with a response rate of 27.5%[40].
However, cisplatin is associated with serious side effects, including renal failure and anaphylaxis. Kawaoka et al[33,41] reported that anaphylaxis occurs more frequently during performance of three or more than three TACE procedure with cisplatin. In recent years, miriplatin has been administered as a third-generation platinum complex that has been developed for hepatic arterial infusion therapy particularly for HCC. Miriplatin {cis-[(1R,2R)-1,2-cyclohexanediamine-N,N’)bis(myristato)]-platinum(II)monohydrate; Dainippon Sumitomo Pharma Co., Ltd., Osaka, Japan} is a novel lipophilic cisplatin derivative that can be suspended in lipiodol. Miriplatin/lipiodol suspension is a stable colloidal emulsion that is deposited within HCC tumors, where active derivatives of miriplatin are gradually released. Also, in a cisplatin-resistant rat hepatoma cell line model, miriplatin did not show cross-resistance with cisplatin[37].
Despite clinical expectations, it is difficult to obtain adequate deposition of miriplatin in HCC, and local recurrences, particularly intra-tumoral recurrences, frequently develop using selective TACE[42]. This may be due to the higher viscosity of miriplatin, since it is suspended in lipiodol; miriplatin may be retained within the artery, so a sufficient amount of the drug does not reach the tumor through narrow blood vessels.
Seko et al[43] reported that reduction of the miriplatin/lipiodol suspension viscosity resistance can be obtained by warming it to 40 °C. Compared to ordinary (room temperature) miriplatin treatment, which has a response rate of 44.3%, efficiency is improved to 70.1% for warmed miriplatin treatment[43]. Kora et al[44] also reported similar treatment outcomes for warmed miriplatin.
Miriplatin is known to have less serious side effects and a lower incidence of renal failure compared to other platinum complex agents. Thus, miriplatin is considered to be suitable for repeat treatments, patients with complications, and elderly patients[45,46].
In recent years, beginning in western countries, permanent spherical embolic material (i.e., beads) have been used in TACE with the aim of more efficient drug delivery[47-49]. Unlike conventional gelatin sponges, the particle size of this material is uniform. Prediction of the level of embolism is straightforward, and a sustained embolic effect can be obtained. It is also possible to impregnate anti-cancer drugs into the beads, and the anti-tumor effect is improved due to the slow release of anti-cancer agents into the tumor.
Two formulations of drug-eluting beads (DEB) are available in Japan: Hepasphere[50] and DC Bead[51] are widely used and each have unique features. DC Bead is a raw material derived from polyvinyl alcohol that is capable of impregnation of positively-charged drugs (e.g., epirubicin, doxorubicin, or irinotecan). Its size is slightly decreased, and its hardness is increased by impregnation of anti-cancer drugs. Meanwhile, Hepasphere is a raw material derived from a polymer with high water absorption and can thus be impregnated with water-soluble anti-cancer agents. The size of Hepasphere increases following impregnation, it expands to about four times its size in the blood, and the resulting embolus is highly flexible and molds to the shape of the target vessel.
In a randomized controlled trial (PRECISION V) that compared TACE using lipiodol (cTACE) to TACE using DC Bead, the complete response rate, objective response rate, and disease control rate were superior in the DC Bead group compared to the cTACE group, although these differences were not statistically significant. In addition, response rates were significantly higher in certain sub-groups, such as in patients with a Child-Pugh score B and in those with HCC in bilateral lobes[52]. Vogl et al[53] also reported that the incidence of decreased left heart ejection fraction, post-embolization liver enzyme elevation, and hepatobiliary system adverse events were lower in the DC Bead group compared to the cTACE group[53]. Sacco et al[54] reported similar results from a randomized controlled trial of DEB-TACE vs cTACE for unresectable HCC: post-treatment elevation of alanine aminotransferase was frequently observed in the cTACE group. However, time to progression and survival did not significantly differ between the two groups: the cumulative 2-year survival rates were 86.8% in the DEB-TACE group and 83.6% in the cTACE group[54].
For TACE with epirubicin-eluting Hepasphere, Seki et al[55] reported a 1-mo response rate of 56.3% and a 6-mo response rate of 52.6%, using response rates as defined by the EASL criteria[55]. For the treatment of HCCs that became refractory to TACE with epirubicin-eluting Hepasphere, changing the impregnated anti-cancer drug to cisplatin resulted in a response at 6 mo in 40% of patients[56].
Several retrospective studies showed the safety and efficacy in DEB-TACE group were significantly higher than in cTACE group (Table 1)[57-59].
However, clear evidence of DEB-TACE superiority compared to cTACE has not been established to date.
There are limitations in this review. First, this is not a systemic review. Therefore, this article may have the potential biases of the authors. Second, we mostly described Japanese history of TACE for HCC in this review.
Improvement of the therapeutic effects of TACE treatment for HCC has been obtained by progression in techniques, drugs, and therapeutic equipment. In a variety of TACE treatments, selecting the anti-cancer agents, treatment methods and equipment for the best therapeutic effect is becoming more important. In the future, it is necessary to clarify the optimal treatment choices for each HCC patient.
P- Reviewer: Lau WY, Li JD, Minami Y S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109:542-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 323] [Article Influence: 32.3] [Reference Citation Analysis (1)] |
| 2. | Ikeda K, Kobayashi M, Kawamura Y, Imai N, Seko Y, Hirakawa M, Hosaka T, Sezaki H, Akuta N, Saitoh S. Stage progression of small hepatocellular carcinoma after radical therapy: comparisons of radiofrequency ablation and surgery using the Markov model. Liver Int. 2011;31:692-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Ikeda K, Kawamura Y, Kobayashi M, Fukushima T, Sezaki H, Hosaka T, Akuta N, Saitoh S, Suzuki F, Suzuki Y. Prevention of disease progression with anti-inflammatory therapy in patients with HCV-related cirrhosis: a Markov model. Oncology. 2014;86:295-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148:397-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 693] [Cited by in F6Publishing: 648] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 5. | Lin DY, Liaw YF, Lee TY, Lai CM. Hepatic arterial embolization in patients with unresectable hepatocellular carcinoma--a randomized controlled trial. Gastroenterology. 1988;94:453-456. [PubMed] [Cited in This Article: ] |
| 6. | Ikeda K, Kumada H, Saitoh S, Arase Y, Chayama K. Effect of repeated transcatheter arterial embolization on the survival time in patients with hepatocellular carcinoma. An analysis by the Cox proportional hazard model. Cancer. 1991;68:2150-2154. [PubMed] [Cited in This Article: ] |
| 7. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2502] [Cited by in F6Publishing: 2483] [Article Influence: 112.9] [Reference Citation Analysis (0)] |
| 8. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1904] [Cited by in F6Publishing: 1903] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 9. | Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 630] [Cited by in F6Publishing: 596] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 10. | Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 580] [Cited by in F6Publishing: 604] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 11. | Ikeda M, Arai Y, Park SJ, Takeuchi Y, Anai H, Kim JK, Inaba Y, Aramaki T, Kwon SH, Yamamoto S. Prospective study of transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: an Asian cooperative study between Japan and Korea. J Vasc Interv Radiol. 2013;24:490-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5972] [Cited by in F6Publishing: 6338] [Article Influence: 487.5] [Reference Citation Analysis (1)] |
| 13. | Arii S, Sata M, Sakamoto M, Shimada M, Kumada T, Shiina S, Yamashita T, Kokudo N, Tanaka M, Takayama T. Management of hepatocellular carcinoma: Report of Consensus Meeting in the 45th Annual Meeting of the Japan Society of Hepatology (2009). Hepatol Res. 2010;40:667-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387-6392. [PubMed] [Cited in This Article: ] |
| 15. | Uchida H, Ohishi H, Matsuo N, Nishimine K, Ohue S, Nishimura Y, Maeda M, Yoshioka T. Transcatheter hepatic segmental arterial embolization using lipiodol mixed with an anticancer drug and Gelfoam particles for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 1990;13:140-145. [PubMed] [Cited in This Article: ] |
| 16. | Demachi H, Matsui O, Abo H, Tatsu H. Simulation model based on non-newtonian fluid mechanics applied to the evaluation of the embolic effect of emulsions of iodized oil and anticancer drug. Cardiovasc Intervent Radiol. 2000;23:285-290. [PubMed] [Cited in This Article: ] |
| 17. | Brown DB, Chapman WC, Cook RD, Kerr JR, Gould JE, Pilgram TK, Darcy MD. Chemoembolization of hepatocellular carcinoma: patient status at presentation and outcome over 15 years at a single center. AJR Am J Roentgenol. 2008;190:608-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Matsui O, Kadoya M, Yoshikawa J, Gabata T, Arai K, Demachi H, Miyayama S, Takashima T, Unoura M, Kogayashi K. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology. 1993;188:79-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 305] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Kawamura Y, Ikeda K, Hirakawa M, Hosaka T, Kobayashi M, Saitoh S, Yatsuji H, Sezaki H, Akuta N, Suzuki F. Efficacy of platinum analogue for advanced hepatocellular carcinoma unresponsive to transcatheter arterial chemoembolization with epirubicin. Hepatol Res. 2009;39:346-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Wallace MJ, Murthy R, Kamat PP, Moore T, Rao SH, Ensor J, Gupta S, Ahrar K, Madoff DC, McRae SE. Impact of C-arm CT on hepatic arterial interventions for hepatic malignancies. J Vasc Interv Radiol. 2007;18:1500-1507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Kakeda S, Korogi Y, Ohnari N, Moriya J, Oda N, Nishino K, Miyamoto W. Usefulness of cone-beam volume CT with flat panel detectors in conjunction with catheter angiography for transcatheter arterial embolization. J Vasc Interv Radiol. 2007;18:1508-1516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Nakamura H, Hashimoto T, Oi H, Sawada S, Furui S, Mizumoto S, Monden M. Treatment of hepatocellular carcinoma by segmental hepatic artery injection of adriamycin-in-oil emulsion with overflow to segmental portal veins. Acta Radiol. 1990;31:347-349. [PubMed] [Cited in This Article: ] |
| 23. | de Baere T, Zhang X, Aubert B, Harry G, Lagrange C, Ropers J, Dufaux J, Lumbroso J, Rougier P, Ducreux M. Quantification of tumor uptake of iodized oils and emulsions of iodized oils: experimental study. Radiology. 1996;201:731-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Takayasu K, Muramatsu Y, Maeda T, Iwata R, Furukawa H, Muramatsu Y, Moriyama N, Okusaka T, Okada S, Ueno H. Targeted transarterial oily chemoembolization for small foci of hepatocellular carcinoma using a unified helical CT and angiography system: analysis of factors affecting local recurrence and survival rates. AJR Am J Roentgenol. 2001;176:681-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Toyoda H, Kumada T, Sone Y. Impact of a unified CT angiography system on outcome of patients with hepatocellular carcinoma. AJR Am J Roentgenol. 2009;192:766-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Miyayama S, Matsui O, Yamashiro M, Ryu Y, Kaito K, Ozaki K, Takeda T, Yoneda N, Notsumata K, Toya D. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol. 2007;18:365-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 27. | Miyayama S, Matsui O, Yamashiro M, Ryu Y, Takata H, Takeda T, Aburano H, Shigenari N. Iodized oil accumulation in the hypovascular tumor portion of early-stage hepatocellular carcinoma after ultraselective transcatheter arterial chemoembolization. Hepatol Int. 2007;1:451-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Miyayama S, Mitsui T, Zen Y, Sudo Y, Yamashiro M, Okuda M, Yoshie Y, Sanada T, Notsumata K, Tanaka N. Histopathological findings after ultraselective transcatheter arterial chemoembolization for hepatocellular carcinoma. Hepatol Res. 2009;39:374-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Golfieri R, Renzulli M, Mosconi C, Forlani L, Giampalma E, Piscaglia F, Trevisani F, Bolondi L. Hepatocellular carcinoma responding to superselective transarterial chemoembolization: an issue of nodule dimension? J Vasc Interv Radiol. 2013;24:509-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Irie T, Takahashi N. Improved accumulation of lipiodol under balloon-occluded transarterial chemoembolization (B-TACE) for hepatocellular carcinoma: measurement of blood pressure at the embolized artery before and after balloon inflation. IVR. 2009;26:49-54. [Cited in This Article: ] |
| 31. | Irie T, Kuramochi M, Takahashi N. Dense accumulation of lipiodol emulsion in hepatocellular carcinoma nodule during selective balloon-occluded transarterial chemoembolization: measurement of balloon-occluded arterial stump pressure. Cardiovasc Intervent Radiol. 2013;36:706-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Sahara S, Tanihata H, Sato M, Kawai N, Takasaka I, Minamiguchi H, Nakai M, Sonomura T. Effects of hepatic artery chemoembolization using cisplatin-lipiodol suspension with gelatin sponge particles on swine liver. J Vasc Interv Radiol. 2009;20:1359-1364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Kawaoka T, Aikata H, Takaki S, Katamura Y, Hiramatsu A, Waki K, Takahashi S, Hieda M, Toyota N, Ito K. Transarterial infusion chemotherapy using cisplatin-lipiodol suspension with or without embolization for unresectable hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2009;32:687-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Osuga K, Arai Y, Anai H, Takeuchi Y, Aramaki T, Sugihara E, Yamamoto T, Inaba Y, Ganaha F, Seki H. Phase I/II multicenter study of transarterial chemoembolization with a cisplatin fine powder and porous gelatin particles for unresectable hepatocellular carcinoma: Japan Interventional Radiology in Oncology Study Group Study 0401. J Vasc Interv Radiol. 2012;23:1278-1285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Kim HC, Lee JH, Chung JW, Kang B, Yoon JH, Kim YJ, Lee HS, Jae HJ, Park JH. Transarterial chemoembolization with additional cisplatin infusion for hepatocellular carcinoma invading the hepatic vein. J Vasc Interv Radiol. 2013;24:274-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Kawamura Y, Ikeda K, Fukushima T, Seko Y, Hara T, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Saitoh S. What Is the Most Effective Drug Delivery System for Cisplatin during the Treatment of Hepatic Tumors with Single-Session Transcatheter Chemotherapy? A Pilot Study. Gut Liver. 2013;7:576-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Imai N, Ikeda K, Seko Y, Kawamura Y, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Saitoh S, Suzuki F. Previous chemoembolization response after transcatheter arterial chemoembolization (TACE) can predict the anti-tumor effect of subsequent TACE with miriplatin in patients with recurrent hepatocellular carcinoma. Oncology. 2011;80:188-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Okusaka T, Kasugai H, Ishii H, Kudo M, Sata M, Tanaka K, Shioyama Y, Chayama K, Kumada H, Yoshikawa M. A randomized phase II trial of intra-arterial chemotherapy using SM-11355 (Miriplatin) for hepatocellular carcinoma. Invest New Drugs. 2012;30:2015-2025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Imai Y, Chikayama T, Nakazawa M, Watanabe K, Ando S, Mizuno Y, Yoshino K, Sugawara K, Hamaoka K, Fujimori K. Usefulness of miriplatin as an anticancer agent for transcatheter arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Gastroenterol. 2011;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Maeda N, Osuga K, Higashihara H, Tomoda K, Mikami K, Nakazawa T, Nakamura H, Tomiyama N. Transarterial chemoembolization with cisplatin as second-line treatment for hepatocellular carcinoma unresponsive to chemoembolization with epirubicin-Lipiodol emulsion. Cardiovasc Intervent Radiol. 2012;35:82-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Kawaoka T, Aikata H, Katamura Y, Takaki S, Waki K, Hiramatsu A, Takahashi S, Hieda M, Kakizawa H, Chayama K. Hypersensitivity reactions to transcatheter chemoembolization with cisplatin and Lipiodol suspension for unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:1219-1225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Miyayama S, Yamashiro M, Shibata Y, Hashimoto M, Yoshida M, Tsuji K, Toshima F, Matsui O. Comparison of local control effects of superselective transcatheter arterial chemoembolization using epirubicin plus mitomycin C and miriplatin for hepatocellular carcinoma. Jpn J Radiol. 2012;30:263-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Seko Y, Ikeda K, Kawamura Y, Fukushima T, Hara T, Sezaki H, Hosaka T, Akuta N, Suzuki F, Kobayashi M. Antitumor efficacy of transcatheter arterial chemoembolization with warmed miriplatin in hepatocellular carcinoma. Hepatol Res. 2013;43:942-949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Kora S, Urakawa H, Mitsufuji T, Osame A, Higashihara H, Yoshimitsu K. Warming effect on miriplatin-lipiodol suspension as a chemotherapeutic agent for transarterial chemoembolization for hepatocellular carcinoma: preliminary clinical experience. Cardiovasc Intervent Radiol. 2013;36:1023-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Imai N, Ikeda K, Kawamura Y, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Saitoh S, Suzuki F, Suzuki Y. Transcatheter arterial chemotherapy using miriplatin-lipiodol suspension with or without embolization for unresectable hepatocellular carcinoma. Jpn J Clin Oncol. 2012;42:175-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Imai N, Ikeda K, Seko Y, Kawamura Y, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Saitoh S, Suzuki F. Transcatheter arterial chemotherapy with miriplatin for hepatocellular carcinoma patients with chronic renal failure: report of three cases. Gut Liver. 2013;7:246-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Bilbao JI, de Luis E, García de Jalón JA, de Martino A, Lozano MD, de la Cuesta AM, Sangro B. Comparative study of four different spherical embolic particles in an animal model: a morphologic and histologic evaluation. J Vasc Interv Radiol. 2008;19:1625-1638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Osuga K, Hori S, Hiraishi K, Sugiura T, Hata Y, Higashihara H, Maeda N, Tomoda K, Nakamura H. Bland embolization of hepatocellular carcinoma using superabsorbent polymer microspheres. Cardiovasc Intervent Radiol. 2008;31:1108-1116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Lencioni R, de Baere T, Burrel M, Caridi JG, Lammer J, Malagari K, Martin RC, O’Grady E, Real MI, Vogl TJ. Transcatheter treatment of hepatocellular carcinoma with Doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol. 2012;35:980-985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 50. | Grosso M, Vignali C, Quaretti P, Nicolini A, Melchiorre F, Gallarato G, Bargellini I, Petruzzi P, Massa Saluzzo C, Crespi S. Transarterial chemoembolization for hepatocellular carcinoma with drug-eluting microspheres: preliminary results from an Italian multicentre study. Cardiovasc Intervent Radiol. 2008;31:1141-1149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 51. | Lewis AL, Gonzalez MV, Lloyd AW, Hall B, Tang Y, Willis SL, Leppard SW, Wolfenden LC, Palmer RR, Stratford PW. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol. 2006;17:335-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 304] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 52. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1063] [Cited by in F6Publishing: 1115] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 53. | Vogl TJ, Lammer J, Lencioni R, Malagari K, Watkinson A, Pilleul F, Denys A, Lee C. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol. 2011;197:W562-W570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 54. | Sacco R, Bargellini I, Bertini M, Bozzi E, Romano A, Petruzzi P, Tumino E, Ginanni B, Federici G, Cioni R. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1545-1552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 55. | Seki A, Hori S, Kobayashi K, Narumiya S. Transcatheter arterial chemoembolization with epirubicin-loaded superabsorbent polymer microspheres for 135 hepatocellular carcinoma patients: single-center experience. Cardiovasc Intervent Radiol. 2011;34:557-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Seki A, Hori S. Switching the loaded agent from epirubicin to cisplatin: salvage transcatheter arterial chemoembolization with drug-eluting microspheres for unresectable hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2012;35:555-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Song MJ, Chun HJ, Song do S, Kim HY, Yoo SH, Park CH, Bae SH, Choi JY, Chang UI, Yang JM. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57:1244-1250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 58. | Wiggermann P, Sieron D, Brosche C, Brauer T, Scheer F, Platzek I, Wawrzynek W, Stroszczynski C. Transarterial Chemoembolization of Child-A hepatocellular carcinoma: drug-eluting bead TACE (DEB TACE) vs. TACE with cisplatin/lipiodol (cTACE). Med Sci Monit. 2011;17:CR189-CR195. [PubMed] [Cited in This Article: ] |
| 59. | Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, Kim HS. Comparison of conventional transarterial chemoembolization (TACE) and chemoembolization with doxorubicin drug eluting beads (DEB) for unresectable hepatocelluar carcinoma (HCC). J Surg Oncol. 2010;101:476-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |