Published online Aug 15, 2014. doi: 10.4239/wjd.v5.i4.444
Revised: January 26, 2014
Accepted: June 20, 2014
Published online: August 15, 2014
Diabetes mellitus is a chronic condition that occurs when the body cannot produce enough or effectively use of insulin. Compared with individuals without diabetes, patients with type 2 diabetes mellitus have a considerably higher risk of cardiovascular morbidity and mortality, and are disproportionately affected by cardiovascular disease. Most of this excess risk is it associated with an augmented prevalence of well-known risk factors such as hypertension, dyslipidaemia and obesity in these patients. However the improved cardiovascular disease in type 2 diabetes mellitus patients can not be attributed solely to the higher prevalence of traditional risk factors. Therefore other non-traditional risk factors may be important in people with type 2 diabetes mellitus. Cardiovascular disease is increased in type 2 diabetes mellitus subjects due to a complex combination of various traditional and non-traditional risk factors that have an important role to play in the beginning and the evolution of atherosclerosis over its long natural history from endothelial function to clinical events. Many of these risk factors could be common history for both diabetes mellitus and cardiovascular disease, reinforcing the postulate that both disorders come independently from “common soil”. The objective of this review is to highlight the weight of traditional and non-traditional risk factors for cardiovascular disease in the setting of type 2 diabetes mellitus and discuss their position in the pathogenesis of the excess cardiovascular disease mortality and morbidity in these patients.
Core tip: The objective of this review is to highlight the importance of traditional and non-traditional risk factors for cardiovascular disease in the setting of type 2 diabetes mellitus and discuss their position in the pathogenesis of the excess cardiovascular disease mortality and morbidity in these patients.
- Citation: Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Cañizo-Gómez FJD. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J Diabetes 2014; 5(4): 444-470
- URL: https://www.wjgnet.com/1948-9358/full/v5/i4/444.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i4.444
Diabetes mellitus (DM) is a chronic condition that occurs when the body cannot produce enough or effectively use of insulin, and are induced by a genetic predisposition coupled with environmental factors[1].
Three hundred sixty six million people have DM in 2011; half of these (183 million people) are undiagnosed[2]. The number of people with DM worldwide is increasing and by 2030 this will have risen to 552 million[2].
DM is a well-established risk factor for cardiovascular disease (CVD). People with type 2 diabetes mellitus (T2DM) have a higher cardiovascular morbidity and mortality, and are disproportionately affected by CVD compared with non-diabetic subjects[3]. Diabetic vascular disease is responsible for two-four-fold rise in the occurrence of coronary artery disease (CAD) and stroke, and two-eight-fold improve in the risk of heart failure[4]. It has been described that patients with T2DM and no previous history of CAD have the similar risk for cardiac events as subjects with a prior myocardial infarction[5]. However, subsequent studies have revealed variable results[6], which more indication that diabetes status may not be a CVD equivalent in all conditions, thus highlighting the necessity for multivariate approach as an suitable basis for risk stratification for CVD prevention in persons with diabetes[7]. The CVD risk follows a gradient, and taking this gradient depends on the combination of numerous risk factors[7]. Most of this excess risk is it associated with an improved prevalence of well-known risk factors such as hypertension, dyslipidaemia and obesity in these subjects. During the recent decade, conclusive evidence has been gathered that treatment of traditional risk factors is of immense importance for patients with T2DM in the reduction of CVD risk[8,9]. The poor control of the majority of cardiovascular risk factors observed in the diabetic population[10] supports the need for more aggressive arrangement of modifiable cardiovascular risk factors, especially in patients with previous CVD. However the improved cardiovascular disease in T2DM patients cannot be attributed solely to the higher prevalence of traditional risk factors. Therefore other non-traditional risk factors may be important in people with T2DM[11] (Table 1). Very few studies have shown prospectively the association of non-traditional risk factors in T2DM, independent of traditional risk factors[12]. Moreover therapies that are currently used in the management of T2DM such insulin-sensitizers and statins have a variety of effects on many of these non-traditional risk factors[13,14]. The relative magnitude of these risk factors has been widely reviewed in the literature[15].
| Traditional | Nontraditional |
| Dyslipidaemia | Insulin resistance and Hyperinsulinemia |
| Hypertension | Postprandial Hyperglycaemia |
| Obesity | Glucose variability |
| Abdominal obesity | Microalbuminuria |
| Physical exercise | Haematological factors |
| Cigarette smoking | Thrombogenic factors |
| Inflammation C-reactive protein | |
| Homocysteine and vitamins | |
| Erectile dysfunction | |
| Genetics and Epigenetics |
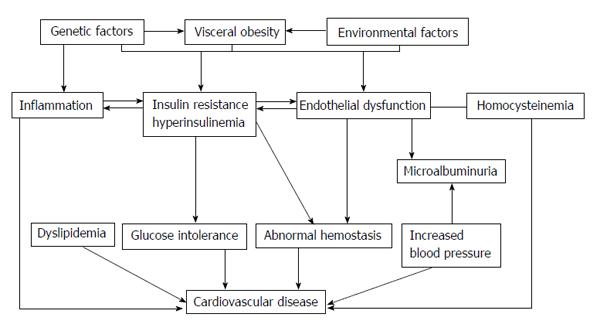
Several studies have aided elucidate the mechanisms underlying the vascular dysfunction that leads to cardiovascular outcomes in DM. This vascular dysfunction is related with visceral adiposity, insulin resistance (IR) and changes in the levels of a diversity of circulating factors[16]. The atherogenesis begins as an endothelial cell dysfunction when various noxious insults as dyslipidaemia, hypertension, diabetes, smoking, etc. induce deficits of nitric oxide (NO) and prostacyclin. Next, mononuclear cells such as monocytes and T lymphocytes binding to the endothelium; this process is mediated by adhesion molecules present on the endothelial surface, such as vascular cell adhesion molecule (VCAM), intercellular adhesion molecule (ICAM) and E-selectin. Monocyte migrates into the sub endothelial space, matures into a resident macrophage and takes up lipid through certain scavenger receptors such as SR-A and CD-36, becomes a foam cell. Later, smooth muscle cells migrate to the surface and form the fibrous cap of the lesion, and lastly lipid-laden macrophages release matrix metalloproteinase’s causing plaque rupture and acute coronary syndromes such as myocardial infarction and unstable angina. Oxidative stress (OE) play an important role in atherogenesis, especially in DM[17,18], by proatherogenic role of oxidized low-density lipoprotein and its “in vivo” existence[19,20]. Elements that may promote increased OE in DM comprise antioxidant deficiencies, increased production of reactive oxygen species and the process of glycation and glyco-oxilation[20]. Increased plasma levels of nitrotyrosine, a marker of protein oxidation[21,22], elevated both plasma and urine levels of F2-isoprostane, a marker of OE[21-23] also the evidence of oxidative damage to DNA[24], was observed in patients with T2DM.
In summary, CVD is elevated in T2DM due to a complex combination of various traditional and non-traditional risk factors, that have an important role to play in the beginning and the evolution of atherosclerosis over its long natural history from endothelial function to clinical events[25]. The clustering of vascular risk observed in association with IR has led to the view that cardiovascular risk appears early, before the development of T2DM, whereas the solid interactions between hyperglycaemia and microvascular disease suggests that this risk is not appear until frank hyperglycaemia appears. These notions highlight the progressive nature of both T2DM and related cardiovascular risk which propose specific challenges at diverse stages of the life of a subject with DM[26]; but do diabetic patients have specific risk factors which could explain the observed increase in CVD, or have all cardiovascular risk factors, traditional and non-traditional, the same strength?
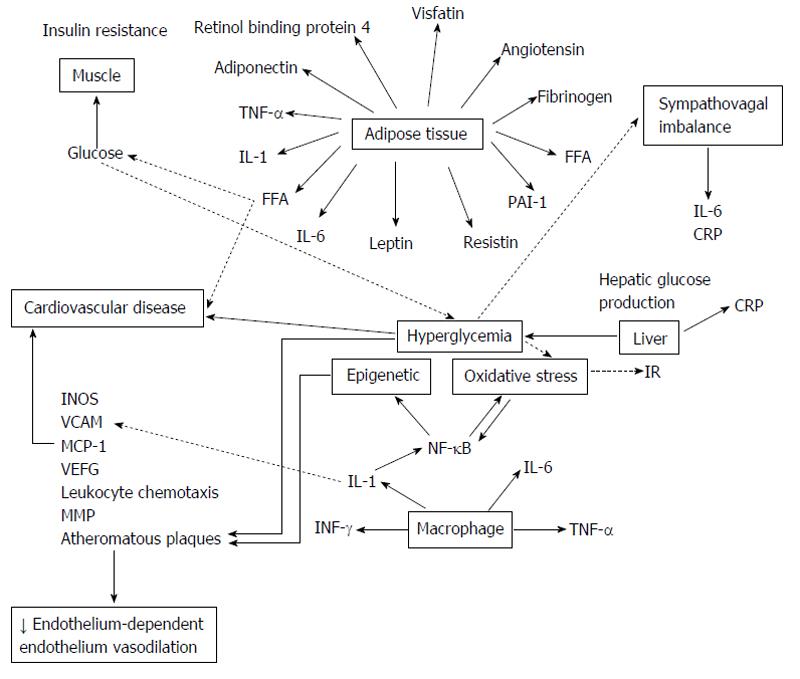
The objective of this review is to highlight the weight of traditional and non-traditional risk factors for CVD in the setting of T2DM and debate their position in the pathogenesis of the excess CVD mortality and morbidity in these patients. It is essential to know that these risk factors do not act in isolation. Risk factors occur simultaneously[27], compounding the risk for a cardiovascular event, although such interactions are difficult to quantify (Figure 1). Many of these risk factors may be common history for both DM and CVD, reinforcing the postulate that both disorders come independently from “common soil”[28].
In T2DM, IR increases the mobilization of free fatty acids from adipose tissue. There are three mechanisms across which there is increased very low-density lipoproteins hepatic production: an increased lipogenesis, an exacerbation of substrate availability, and decreased apolipoprotein B-100 (ApoB) degradation. These changes carry to a lipid profile marked by low high-density lipoprotein cholesterol (HDL-C), high triglycerides (TGs), increased ApoB synthesis and small dense LDL particles[29]. This LDL subtype is more inclined to oxidation, playing an important role in atherogenesis. Stronger than LDL cholesterol, a low HDL-C or lonely elevated TGs, atherogenic dyslipidaemia (Low HDL-C and ApoA, Elevation of both fasting and post-prandial TGs, Elevation of small dense LDL particles, Elevation of ApoB) is in T2DM patients a self-determining predictor of cardiovascular risk. The protective function of HDL may be lost in type 2 diabetics owing to alterations of the protein, resulting in a pro-oxidant, inflammatory phenotype[30].
Association between dyslipidaemia and cardiovascular risk in T2DM: A causal association exists between elevation of TGs-rich particles and their remnants, low HDL-C and cardiovascular risk[31,32] as is shown in large data from case-control, genetic, and large observational studies. Still in patients with a normal LDL-C levels, results from statin trials confirm the place of low HDL as an independent cardiovascular risk marker[33,34]. Cardiovascular event rates were significantly greater in those with dyslipidaemia: LDL-C > 2.6 mmol/L, HDL-C ≤ 0.88 mmol/L and TGs ≥ 2.3 mmol/L[35,36], as is proved in the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study and in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study. The FIELD study[37] defined the following variables as best predictors of cardiovascular events during a five year monitoring: lipid ratios non-HDL/HDL-C and total/HDL-C. Ratio of ApoB/ApoA is also associated to CVD outcomes, but this ratio wasn’t superior to conventional lipid ratios. Data from the Emerging Risk Factor Collaboration (ERFC) study[38] with 302430 persons with no history of cardiovascular disease, demonstrated that Apo B and non-HDL-C each had very similar association with coronary heart disease (CHD) regardless of the existence of diabetes. The ERFC study showed that an increase of 0.38 mmol/L or 15 mg/dL in HDL-C was associated with a 22% reduction in risk of CHD. Non-HDL-C was the best tool to define the risk linked with TGs rise in clinical practice[38].
Management of dyslipidaemia, significance in the prevention of CVD in T2DM: As the development of atherogenic dyslipidaemia precedes the onset of overt glycaemia and the clinical diagnosis of diabetes, early effective intervention is recommended to reduce the risk of premature CVD.
In T2DM large data exists on action mechanism and efficacy of statins in the prevention of CVD events[39]. The Collaborative Atorvastatin Diabetes Study assessed the benefits of a statin in T2DM patients and at least one of the following risk factors: albuminuria, retinopathy, hypertension or current smoking[40]. In this study, 2838 type 2 diabetics were randomized to placebo or atorvastatin 10 mg/d. The study was finished ahead of time, because to a 37% reduction (P = 0.0001) in the primary endpoint (first acute CHD event). In the Heart Protection Study, simvastatin (40 mg/d) reduced the composite primary endpoint by 33% (P = 0.0003)[41]. This study was performed with 2912 patients (mainly T2DM) without pre-existing CVD. Also, atorvastatin 10 mg decreased the rate of major CVD events in 23% in the Anglo-Scandinavian Cardiac Outcomes Trial subgroup. Diabetic patients were free from CVD[42].
Residual risk in people on LDL-lowering therapy: Patients with T2DM at the LDL-C target are still at a significant risk of CVD events[31]. This residual risk is associated to several factors as increased on TGs-rich proteins, decreased HDL-C and small, dense LDL particles. Data of FIELD study demonstrated that fenofibrate therapy did not decrease the primary endpoint (non-fatal myocardial infarction and CAD-related death), but total CVD events were decreased from 14% to 12.5% (P = 0.035)[35,43]. However, a subgroup analysis of dyslipidaemic people (TGs > 2.3 mmol/L and HDL-C ≤ 0.9 mmol/L) in this study showed a 27% reduction in CVD risk[35]. In the ACCORD trial, 5518 patients were allocated to fenofibrate plus simvastatin (20-40 mg daily) or placebo without any additional effect on the primary endpoint. In a pre-specified subgroup analysis of people with TGs > 204 mg/dL and HDL-C < 34 mg/dL, cardiovascular risk was decreased in 31% in the fenofibrate-plus-simvastatin group[44]. In both ACCORD and FIELD, treatment with fenofibrate was related with a strong reduction of TGs (22%), whereas increase of HDL-C remained less than expected (2% and 2.4%, respectively). The clinical benefits of fibrates on major CVD events have been confirmed in meta analyses; but not on cardiovascular mortality[43,44]. The effects seem to be appeared to an improvement in TGs[45].
Arterial hypertension is present in more than 60% of T2DM patients[46]. This is directly linked to: (1) increased renin-angiotensin-aldosterone system activity; (2) hyperinsulinemia associated to increased renal reabsorption of sodium; and (3) increased sympathetic tone[47]. Aging, obesity, and the onset of renal disease also promote an increase in the prevalence of hypertension. Hypertension and DM are additive risk factors for CVD. While the diagnosis of diabetes doubles the cardiovascular risk in men and more than triples the risk in women, hypertension quadruple cardiovascular risk in diabetic patients[5,48].
Treatment targets: Lowering blood pressure (BP) under 140 mmHg systolic and 85 mmHg diastolic (Table 2) have shown positive effects on cardiovascular outcomes in randomized controlled trials[49-52]. The United Kingdom Diabetes Prospective Study (UKPDS) showed that strict (mean 144/82 mmHg), compared with less strict (mean 154/87 mmHg) control decreased macrovascular events by 24%. DM-related mortality decreased 15% with each 10 mmHg drop, down to a systolic BP (SBP) of 120 mmHg, with no indication of further decrease, as it was shown in a post-hoc observational analysis of the UKPDS trial[53]. Later, the ACCORD trial doesn’t support a decrease of SBP below 130 mmHg[50].
| Recommendations | Class | Level |
| Blood pressure control is recommended in patients with diabetes mellitus and hypertension to lower the risk of cardiovascular events | I | A |
| It is recommended that a patient with hypertension and diabetes mellitus is treated in an individualized manner, targeting a blood pressure of < 140/85 mmHg | I | A |
| It is recommended that a combination of blood pressure lowering agents is used to achieve blood pressure control | I | A |
| A RAAS blocker (ACE-I or ARB) is recommended in the treatment of hypertension in diabetes mellitus, particularly in the presence of proteinuria or microalbuminuria | I | A |
| Simultaneous administration of two RAAS blockers should be avoided in patients with diabetes mellitus | III | B |
Recent evidence suggests visit-to-visit variability in SBP and masked hypertension are predictors of cardiovascular disease in T2DM.
Effects of visit-to-visit variability in SBP on CVD in T2DM patients[54]: Using the data from ambulatory BP monitoring, previous studies reported that short-term or circadian variability of BP was an important prognostic factor of cardiovascular outcomes[55-58]. Similarly, a number of observational studies have investigates the impact of long-term or visit-to-visit BP variability on the risks of cardiovascular outcomes[59-63]. In the Blood Pressure-Lowering Arm of the Anglo-Scandinavian Cardiac Outcomes Trial, Rothwell et al[59] reported that visit-to-visit SBP variability was a strong predictor of CVD among patients with transient ischemic attack or stroke and among hypertensive patients. In the Action in Diabetes and Vascular Disease (ADVANCE) Trial, which included 8811 patients, visit-to-visit SBP variability was clearly associated with myocardial infarction and cardiovascular death. Another new and important finding of this analysis was that visit-to-visit variability of SBP clearly predicted the future development of major microvascular complications among patients with T2DM[54].
Risk associated with masked hypertension in T2DM patients[64]: Masked hypertension (MH) is defined as an ambulatory hypertension with a normal conventional BP (CBP).
The International Database on Ambulatory BP (ABP) in relation to Cardiovascular Outcomes[65], which contain a great number of diabetic patients, many of whom have MH, detected a higher prevalence of MH in DM than in non DM, and this finding was even more remarkable in treated vs non treated diabetics. Currently is not known the mechanism by which antihypertensive treatment is linked with a higher prevalence of MH. Cardiovascular risk in diabetic patients who are not receiving antihypertensive treatment and presenting with MH was significantly higher than in their normotensive comparator group. In contrast, antihypertensive-treated diabetics with MH had cardiovascular risk that was identical to treated stage 1 and stage 2 hypertensive subjects. This suggests that a significant percentage of these subjects had real hypertension that simulated MH in the presence of elevated ABP and normalized CBP[65].
Nevertheless, currently, there aren’t credible studies in diabetics with MH to evidence the benefit of antihypertensive therapy or to indicate how low to go with the reduction in ABP to achieve optimal reduction in cardiovascular risk. We may have to balance the potential advantage of further reduction in systolic ABP and CBP values with the increased cardiovascular risk of lower diastolic ABP and CBP.
Generalised obesity assessed by the body mass index (BMI), and abdominal obesity determined by the waist circumference (WC), are related with a variety of CVD risk factors. Clinical guidelines do not indicate whether BMI or the WC measurements have identical utility in predicting cardiovascular risk in individuals with T2DM compared to non-diabetic patients[66,67].
The impact of obesity on both atherogenesis and in novel procoagulant and prothrombotic cardiovascular risk factors is of particular interest in cases of T2DM, as they contribute to increased CVD mortality in these individuals[68-72].
In diabetic patients the coexistence of multiple variables such as diabetic duration, glycaemic control and the drugs used for achieving it, lipid profile, BP or the existence of risk behaviours such as smoking or alcohol use may confound the impact of obesity on the risk of CVD[73].
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) study[73] was designed to establish the association between indexes of obesity and atherothrombotic risk factors in patients with T2DM and document CVD. By only taking into account this study’s baseline it was possible to evaluate among this group of patients if a higher BMI or higher WC was associated with specific cardiovascular risk factors, and whether a higher WC was related with cardiovascular risk factors independent of diabetic patient’s BMI. The review of the study baseline results showed, on the one hand, that patients with BMI ≥ 40 experienced more cases of heart failure. However, a history of myocardial infarction was less common in patients with BMI ≥ 35 (26%-30%) than in those with BMI ≤ 29.9 (34%-36%), possibly because patients with BMI ≥ 35 reported fewer years smoking than those with BMI of ≤ 29.9. Smoking was proportionally, inversely related to BMI. Furthermore the BMI, independent of the WC, had a strong association with SBP, the plasminogen activation inhibitor type 1 (PAI-1), the C-reactive protein (CRP) and fibrinogen, whereas WC had robust associations with the HDL-C and TGs levels.
It is well known that CVD is among the most frequent causes of mortality for diabetics and obese individuals. Studies have established the mortality risk in obese T2DM subjects taking the age into account. The data obtained from a study conducted in Verona with 3398 T2DM patients who were followed up for 10 years showed that in patients > 65 years a moderate excess weight predicted longer survival, whereas obesity was a negative prognostic factor in patients < 65 years[74].
On the other hand, the ADVANCE study compared the association between cardiovascular risk and BMI, WC, and the waist to hip ratio in 11140 T2DM patients, and reached the conclusion that the waist to hip ratio is the best predictor of cardiovascular events and mortality in diabetics[75].
Regularly practicing physical exercise is correlated with a lower risk of cardiovascular morbidity and mortality, both in primary and in secondary prevention. However it should be taken into account that this type of evidence is often subject to other lifestyle changes that take place together with exercise (for example stopping smoking, a balanced diet, etc.)[76,77].
Multiple observational studies, conducted in diabetic patients, support that stated above. One such case is an American prospective cohort study of 2896 T2DM adults which showed that those who walked at least two hours per week had lower frequency of CVD mortality compared with inactive patients (HR = 0.66; 95%CI: 0.45-0.96; 1.4% vs 2.1% per year, respectively), and that the risk was even lower for those who walked 3 or 4 h a week. In this study the protective effect of exercise was independent of gender, age, race, BMI, diabetes duration, coexisting comorbidities and physical limitations. The authors estimated that one death per year would be prevented for every 61 individuals with diabetes who were persuaded to walk at least two hours per week[78]. The same occurred in a Finnish study, with 3316 diabetic patients, who showed that physical activity at work and during leisure time was linked with a decrease in cardiovascular mortality and total mortality[79].
It is important to note that patients with T2DM have a reduced capacity for exercise due to age, the high BMI and the frequent presence of left ventricular dysfunction[80]. Exercise improves insulin sensitivity in diabetic patients in the same way as it does in non-diabetic patients[81-83]. Patients with diabetes have greater IR which can be mediated by different defects in the glucose metabolism, and some of which would improve with physical exercise. These defects include not only a decreased number of insulin receptors and glucose transporters, but also a reduction in the intracellular enzymes activity (pyruvate dehydrogenase and glycogen synthase) and reduced oxygenation during exercise. Increased physical activity achieves higher mitochondrial enzyme activity and increases insulin sensitivity; however the number of muscle capillaries in diabetic patients with microvascular complications does not increase or is practically negligible[84-86].
Multiple studies have shown physical exercise to improve cardiovascular risk factors (dyslipidaemia, hypertension and body composition) in patients with T2DM[87]. Although it is not all kinds of physical activity exert the same influence on this risk. Aerobic exercise only or combined with resistance exercise improves glycaemic control, BP, the amount of TGs and WC. But resistance exercise alone does not have a clear impact on cardiovascular risk factors.
In prospective cohort studies, exercise was associated with improved CVD and reduced cardiovascular mortality and total mortality in patients with T2DM[88]. Results from the Nurses’ Health Study[89] reported that 5125 women with T2DM who exercised for at least 4 h per week had a 40% lower risk of developing CVD (comprising heart disease and stroke) compared to those who did not. This risk improvement remained after adjustments for smoking, BMI, and another cardiovascular risk factors.
Smoking is linked with deterioration in metabolic control in diabetic patients[90,91], which is associated with an increased risk for development of macrovascular and microvascular complications and mortality in DM[92,93].
The suggested mechanisms for the influence of smoking on risk of T2DM are summarized in Table 3. Administration of nicotine rise the circulating levels of insulin-antagonistic hormones (growth hormone, catecholamines and cortisol)[94-97], and also has been proved to affect the autonomic nervous system[98,99]. Nicotine, via these and possibly also other mechanisms, decreases insulin sensitivity, directly or indirectly. Also smoking increases circulating free fatty acid levels[95], and this is an additional negative factor for the insulin-mediated glucose uptake[100].
| Direct effects due to inhalation of smoke from tobacco products |
| Impaired insulin sensitivity based on influence of haemodynamic dysregulation in capillary vascular bed |
| Impaired insulin sensitivity due to increase in inflammatory markers secondary to bronchitis and pulmonary infections caused by smoking |
| Impaired beta-cell function due to toxic effects of tobacco smoke |
| Lipotoxicity due to influence of increased triglyceride levels |
| Hypercortisolaemia and increase in abdominal fat tissue |
| Elevated sympathetic nervous activation |
| Indirect effects on glucose metabolism |
| Unhealthy lifestyle in smokers (poor diet, lack of physical activity) |
| Increased alcohol consumption (toxic effects on beta cells) |
| Psychosocial stress and impaired sleep associated with smoking |
| Impaired fetal growth in smoking pregnant women, associated with increased diabetes risk in offspring in adult life |
Smoking and macrovascular complications in T2DM: CVD is responsible for the main proportion of mortality associated with T2DM. There is evidence that smoking improves the risk of CAD in T2DM. Based on data from 4540 patients with T2DM followed in the UKPDS, smoking was shown to rise the risk of CHD[101] in males and females with T2DM. The expected RR incidence of a fatal or non-fatal myocardial infarction or sudden death attributable to smoking was 1.350 (95%CI: 1.11-1.59). This study reveals that smoking is an independent and significant risk factor for stroke[102] and peripheral vascular disease[15].
However, it was proved that smoking is significantly related with an augmented risk for CHD, but not for stroke, in T1 and T2DM patients in the London cohort of the prospective (8-year follow-up) World Health Organization Multinational Study of Vascular Disease in Diabetics[93].
In a prospective cohort of female nurses with T2DM[103], cigarette smoking was found to be robustly associated with the risk of CHD, and this risk improved with the number of cigarettes smoked per day. Compared with the nurses who had never smoked, the RR for CHD was 1.21 (95%CI: 0.97-1.51) for past smokers; 1.66 (95%CI: 1.10-2.52) for current smokers of up to 14 cigarettes per day; and 2.68 (95%CI: 2.07-3.48) for current smokers of 15 cigarettes per day or more.
A relatively large prospective study examined the effects of smoking cessation on cardiovascular risk in diabetic patients[104]. Data from this study reveal that stopping smoking decreases mortality risk in diabetes, but risks keep increased some years after stopping and are highly dependent on the duration of smoking.
Diabetic patients who are current smokers should be proposed a planned smoking cessation program that includes pharmacological treatment if is necessary. Detailed instruction should be provided according to the five A principles (Table 4) as is developed in the 2012 Joint European Prevention Guidelines[105].
| A-ASK: | Systematically inquire about smoking status at every opportunity |
| A-ADVISE: | Unequivocally urge all smokers to quit |
| A-ASSESS: | Determine the person’s degree of addiction and readiness to quit |
| A-ASSIST | Agree on a smoking cessation strategy, including setting a quit date, behavioral counseling, and pharmacological support |
| A-ARRANGE | Arrange a schedule for follow-up |
IR is a principal characteristic of T2DM and it develops in multiple organs involving the skeletal muscle, liver, adipose tissue and the heart. The onset of hyperglycaemia and diabetes is often preceded by several years of IR. Obesity plays a major role in this phenomenon and provides an important link between T2DM and the accumulation of fat[106]. A significant section of the population with T2DM is obese[107].
The hyperinsulinemia, as a result of IR, occurs even before the onset of DM, and could be, by chance, related to vascular disease[108-111].
The IR, measured by the hyperinsulinaemic-euglycemic clamp, or surrogate methods such as the HOMA index, the frequently-sampled intravenous glucose tolerance test or the insulin suppression test, appears in more than 76% of subjects, and is accompanied by compensatory hyperinsulinemia[112]. Although molecular mechanisms of IR are not yet entirely understood, abnormalities in insulin signalling have been explained[113]. Under normal conditions, insulin starts its action by binding to its specific cell surface receptor in peripheral tissues such as liver and skeletal muscle. The conformational changes of the insulin receptor induced by insulin binding to the extracellular alpha-subunit of the insulin receptor, causes the dimerization of neighboring receptors and the activation of the tyrosine kinase domain of the intracellular beta-subunit. Autophosphorylation of the beta-subunit itself, promoted by the onset of tyrosine kinase activity of insulin receptor, and the rapid phosphorylation of docking proteins, such as insulin receptor substrates -1, -2, -3 and -4, and some other proteins, comprising collagen homology proteins (shc) and SRC homology 2 (SH2), activates consecutively multiple intracellular signalling intermediates. In their phosphorylated forms, these proteins develop points of anchoring for intracellular proteins containing complementary SH2 domains, playing an important regulatory function in the insulin-signalling cascade. Specifically, the activation of Akt or protein kinase B, which plays an essential role in the mechanism of insulin action on GLUT-4 translocation, glucose transport, and the activation of NO synthase (“metabolic signalling pathway”), is determined by the interaction between insulin receptor substrate-1 proteins and phosphatidylinositol (PI) 3-kinase. On the contrary, the activation of Ras (predominantly through shc and, to a lesser degree, insulin receptor proteins), Raf, and mitogen-activated protein kinases (MAPK) (“growth signalling pathway”) are implicated in the mitogenic, nonmetabolic, pro-inflammatory and proliferative effects of insulin[114]. A decreased activation of insulin signalling via the insulin receptor substrate-1/PI3-kinase (PI3K), can be showed in insulin-resistant animals and in vitro models. This reduction leads to a decreased glucose uptake, diminished NO synthesis, and reduced glucose utilisation in insulin target tissues pathway. Similar decrease in glucose transport is detected in the pancreatic beta cells, which induces a compensatory rise in insulin secretion. In spite of this, the MAPK-mediated insulin pathway persists unaffected. Under these conditions of hyperinsulinemia, this selective imbalance of the two signal transduction pathways can lead to a disproportionate proliferative/growth-promoting signal, while the normal transport of glucose and glucose homeostasis is conserved. Compensatory hyperinsulinemia stimulates in vascular smooth muscle and endothelial cells, an increased production of endothelin, PAI-1, proinflammatory cytokines and an augmented surface expression of adhesion molecules[115-118].
Homeostasis of blood vessels is conserved through the activation of endothelium-derived NO, stimulated by insulin. By rapid posttranslational mechanisms, which are mediated through PI3K/Akt signaling pathway, insulin augments the endothelial NO production by activating endothelial NO synthase III (endothelial NOS)[119]. In IR states, the selective inhibition of the PI3K/Akt pathway detected in skeletal muscle from obese people and subjects with T2DM[120], and in the vasculature and the myocardium of obese Zucker rats, leads to endothelial dysfunction, with a consequent rise in the interaction between endothelial cells and leukocytes, an increase in vascular tone and BP, and a prothrombotic state. In this selective state, largely due to the ability of insulin to increase NO production, its physiological anti-atherogenic effects become proatherogenic[121].
Postprandial hyperglycaemia has been appeared to be related with an augmented risk of cardiovascular events in patients with and without T2DM[122-125]. Postprandial glucose excursions, especially when accompanied by increased postprandial TGs levels, are pathophysiologically related to augmented OE, systemic inflammation and endothelial dysfunction, all of which are associated to increases in atherosclerosis and cardiovascular events[126,127]. Postmeal hyperglycaemia is also linked to retinopathy, cognitive dysfunction in old people and specific cancers[128]. Relevantly, even in the setting of controlled fasting glucose levels, postprandial spikes in glucose powerfully improve both atherogenesis and cardiovascular events[122-125,129].
Two studies have examined the predictive strength of postprandial glycemia on cardiovascular events. The Intervention Diabetes Study[130], a prospective population-based multicentre trial, conducted in 1139 subjects, aged 30-55 years, newly diagnosed of T2DM, followed up for 11 years; showed that postprandial blood glucose was an independent predictor for death. However, this study did not consider HbA1c. On the other hand, the San Luigi Gonzaga Diabetes Study[122], conducted in 505 T2DM patients followed up for 14 years, indicated that both postprandial blood glucose and HbA1c predict cardiovascular events and all-cause mortality, showing the independent predictive power of postprandial glycemia on cardiovascular events after correction for HbA1c.
It has been shown that intensive control of hyperglycemia prevents macrovascular events and all-cause mortality in individuals with T2DM. A meta-analysis of 5 randomized controlled trials showed that, in T2DM subjects, intensive glycaemic control considerably decreases coronary events without an increased risk of death [131]. However, the specific effect of postprandial blood glucose control on cardiovascular events and mortality, is less clear. The following evidence is available: (1) Intervention with acarbose, a drug that diminish postprandial blood glucose excursions by delaying carbohydrate digestion in the small intestine, can prevent myocardial infarction and CVD in T2DM patients[132]. Moreover, in patients with impaired glucose tolerance, in Study to Prevent Non Insulin Dependent Diabetes Mellitus, acarbose was associated with a 49% relative risk reduction in the development of cardiovascular events[133]; (2) Nateglinide, a drug that lowers postprandial blood glucose by stimulating insulin secretion from the pancreas, was incapable in the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research trial[134] to diminish cardiovascular events among persons with impaired glucose tolerance and established CVD; however, patients on Nateglinide presented an increase of 2-h postchallenge blood glucose[134]; and (3) The Hyperglycemia and its Effect after Acute myocaRdial infarcTion on cardiovascular outcomes in patients with T2DM trial, planned to compare the effects of prandial vs fasting glycemic control on risk for cardiovascular outcomes in subjects with T2DM after acute myocardial infarction, revealed that treating diabetic survivors of acute myocardial infarction with two distinct insulin regimens (prandial vs basal) achieved differences in fasting blood glucose, less-than-expected differences in postprandial blood glucose, and no difference in risk for future cardiovascular event rates[135].
Therefore, the role of postprandial glycemia as a predictor of cardiovascular events, and its importance as a treatment target, are issues to discuss.
These assessments had led to the concept of glucose variability. Recently, it has been suggested that blood glucose variability may contribute, even more than HbA1c, to the development of diabetes complications. However, the lack of consensus on the best approach to define the glucose variability, and difficulty of measuring it, are still unsolved problems. The relationship between glucose variability and OE, is an important physiopathological element for the development of the cardiovascular complications of diabetes. Glucose variability, thus, looks set to become the main target for future treatments for diabetes, aimed to reaching better efficacy in the metabolic control of diabetes and the prevention of complications related to it[136].
The term microalbuminuria (MA), a urinary albumin excretion between 30 and 300 mg/24 h, has been introduced to identify subjects at increased risk of early cardiovascular death and progressive renal disease. In individuals with T2DM, MA is a prematurely clinical sign suggestive of vascular damage to the glomerulus. MA has also been currently reported as an important risk factor for CVD and remains the main and most widely used marker of diabetic renal damage in clinical practice. It is also a marker of organ dysfunction, and has been appeared to be associated with an increased risk of cardiovascular morbidity and mortality in T2DM patients[137]. At present, an increased albumin excretion is considered to be a renal symptom of generalized endothelial dysfunction[138]. According to different studies, the prevalence of MA is up to 19% in T2DM[139-142].
The epidemiology of MA shows a close association with systemic and glomerular endothelial dysfunction and with vascular disease. Damage to glycocalyx, a protein rich surface layer on the glomerular endothelium, probably represents the initial step in the development of diabetic MA[143].
MA is a marker for diabetic nephropathy. It also signifies CVD as well as nephropathy in T2DM. MA may precede T2DM, and forms one of the components of the IR/metabolic syndrome which confer a particularly high risk of cardiovascular deaths. Therefore, MA accounts for the increased risk of vascular disease in subjects with metabolic syndrome[144]. Other indicators of cardiovascular risk, such as markers of inflammation, are related with MA in population of patients with and without diabetes[145]. The existence of MA in people with T2DM is the most important early sign that we alert us to the onset of a systemic vascular disease, and associated target organ damage to the heart, the brain and the kidney. Their presence serves to recognize patients at risk of early cardiovascular death and advancement of kidney disease[146].
Patients with MA are at very high vascular risk and should share identical objectives of a vascular risk factor control as patients with overt CVD[147]. MA in patients with T2DM positively correlates with the severity of coronary atherosclerosis[148]. Reinhard et al[149] showed that half of asymptomatic patients with T2DM and MA, which received an intensive multifactorial treatment for cardiovascular risk diminution, had significant atherosclerosis in at least one vascular territory. They observed a higher prevalence of coronary atherosclerosis than carotid disease[149]. On the other hand, MA was higher in T2DM patients with silent myocardial ischemia[150].
The presence of MA also indicates that a low-level inflammatory process is ongoing. In hypertensive individuals, with or without diabetes, increasing MA is related with augmented levels of inflammatory markers, endothelial dysfunction and platelet activation[151]. Elevated plasma osteoprotegerin, a cytokine receptor, is an independent predictor of the presence of CVD in asymptomatic T2DM patients with MA[152], and CRP, a marker of inflammation, was an independent risk factor for development of nephropathy in T2DM patients[153]. Finally, D-dimer, a fibrin degradation product, is associated with MA in T2DM patients; this suggests that glomerular dysfunction is in part mediated by hypercoagulability[154].
Duration of diabetes[139], diabetes severity[139], uncontrolled hypertension[139,141,153,155-157], baseline levels of urinary albumin excretion > 12 mg/24 h[153], BMI[139,157], central obesity[139,140,155], high HbA1c[139,141,157], smoking habits[140,155,157], age of the patients[156], creatinine[141], CRP > 3 mg/L[153], as well as TGs and HDL-C[136,156] were independent risk factors for the development of MA in T2DM patients. These risk factors were independently associated with established MA. Population of normotensive subjects with T2DM and MA, female sex, was related with elevated risk of fatal and nonfatal CVD, independent of the traditional cardiovascular risk factors, the severity of nephropathy or existence of retinopathy, or health care utilization[158]; and a decreased estimated glomerular filtration rate and the occurrence of MA were each related with a near doubling of the prevalence of CVD, independently of classical cardiovascular risk factors and glycaemia control in subjects with T2DM[159].
Carotid stiffness, quantified by quantitative carotid stiffness, a local functional measurement of the arterial wall, is augmented in T2DM patients with MA[160]. MA is also independently linked with arterial stiffness and vascular inflammation in individuals with newly diagnosed T2DM[161], but not with carotid intima-media thickness[161,162], with emphasizes the significance of proactive clinical investigations for atherosclerotic complications in subjects with MA in newly diagnosed DM. On the other hand, patients with MA have more severe angiographically detected CAD than those without MA[163]. Thus relative is independent of other risk factors and is particularly evident in patients with T2DM[164].
In conclusion, MA is a marker for diabetic nephropathy. It also signifies CVD in T2DM. MA is predictive, independent of classical risk factors and all causes of mortality in T2DM individuals. Determination of MA has been shown to be helpful to recognize patients with T2DM at high risk of renal and CVD. MA is correlated with higher cardiovascular mortality, especially in diabetics, but the direct relationship between MA and vascular wall properties is still not clear.
Atherothrombosis, defined as the formation of a thrombus on a pre-existing atherosclerotic plaque, is the leading cause of mortality in the Western world. Diabetes has been recognised as an independent risk factor and atherothrombosis accounts for the 80% of deaths in these patients[165,166]. It is the result of the progression of atherosclerosis, and its major manifestations are sudden cardiac death, myocardial infarction, stroke and peripheral arterial ischemia[167].
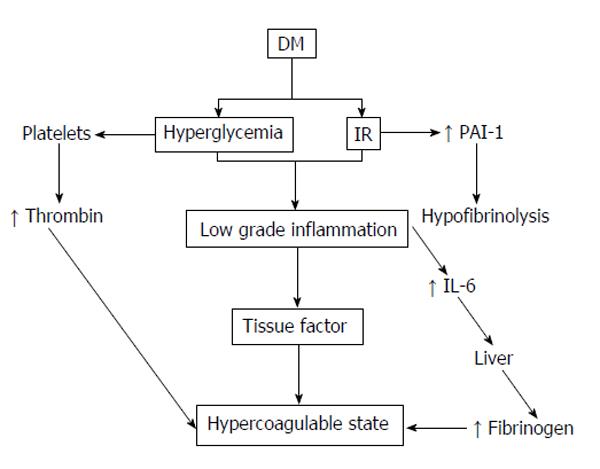
Diabetes is related with a hypercoagulable state, which is more pronounced during the postprandial period. Hyperactivated platelets at injured endothelial interfaces act, together with an improved availability of thrombotic precursors, decreased coagulation inhibitors and diminished fibrinolysis[168]. The UKPDS clearly showed that macrovascular events in patients with T2DM accounted for more than 50% of total mortality[169]. Atherosclerosis develops more quickly and aggressively in diabetes, and leads more frequently to thrombotic events due to the interaction between the vascular wall and hypercoagulability[170,171].
In DM, the activation of the intrinsic coagulation pathway occurs more easily and fibrinolysis diminishes[172]. The increased platelet activity signifies increased adhesion and aggregation in diabetic patients (Figure 2). Individuals with various stages of diabetes were showed to have increased numbers of CD62P-positive and CD63-positive platelets (activated platelets) compared to healthy subjects. This increase in circulating activated platelets is not associated with glycaemic control improvement thereby intensifying insulin therapy. Surprisingly this increase in CD62P-positive platelets can also be found in healthy, first-degree relatives of patients with T1DM. Additionally, significant increments in basal thromboxane B[164] are seen in the platelets of both type T1 and T2DM, both in patients with an absence of vascular complications, as well as those with good diabetic control.
Flow cytometry has revealed that a large, hyperactive platelet subpopulation circulates in patients with DM, at a similar level to patients who have experienced a myocardial infarction. This suggests that the increased aggregation potential of these platelets lowers their activation threshold, thus contributing to the augmented incidence of acute cardiovascular events in DM[173].
Apart from platelet hyperactivity, DM also predisposes the coagulation system to other disorders[174]. Fibrinolysis is a natural defence system against thrombosis. Under physiological conditions, there is a balance between plasminogen activators and inhibitors; however, an imbalance can be caused by a reduction in the tissue plasminogen activator levels or an increase in the PAI-1 levels. This prethrombotic state in diabetic patients has been explained by multiple hypotheses. One such hypothesis is based on various studies showing the high levels of PAI-1 found in diabetic patients[175,176]. High concentrations of PAI-1 have been implicated with an increase in cardiovascular morbidity and mortality with age. A search was made for the relation of PAI-1 with various factors such as age, gender and ethnicity in subjects with T2DM and stable CAD enrolled in the BARI 2D study. The results of this study concluded that in subjects with T2DM and stable CAD, the levels of PAI-1 antigen and its activity were paradoxically lesser with advancing age; and in contrast, D-dimer (P < 0.0001) was increased, revealing elevated fibrinolysis. These results may indicate a protective phenomenon resulting in an improved survival in some older people with DM that endowed them with longevity enough to permit them to participate in the BARI 2D study[177].
Another hypothesis in this prethrombotic state is that hyperglycaemia permits the protein glycosylation process, such as the fibrinogen, which affects the clot’s physiological structure, and thus it is more resistant to plasmin degradation.
DM is also associated with increased plasma fibrinogen, which is considered as another cardiovascular risk factor[12,178]. This increase in fibrinogen is also associated with other vascular risk factors such as old age, increased BMI, smoking, total cholesterol and TGs. Fibrinogen has been extensively studied by many researchers, and a connection between the amount of fibrinogen and fibrin present in the vascular wall, the fibrinogen plasma concentration and the severity of atherosclerosis has been established. This association has been shown to be more evident in patients with diabetes[179,180]. Furthermore, an elevated concentration of fibrinogen has been found in diabetic patients with albuminuria. Some authors believe that the increased levels of fibrinogen, factor VII and von Willebrand factor which have been found in DM patients serve as predictors of coronary atherosclerosis and cardiovascular risk factors[181]. This association supports the fact that diabetic patients develop cardiovascular complications more frequently than the healthy population.
Atherosclerotic CHD and other forms of CVD are the main cause of mortality in T2DM, as well a major contributor to morbidity and lifetime costs. When diabetes occurs in subjects with established CAD, absolute risk for future events is very high. Inflammation has been involved in the pathogenesis of CVD, T2DM, and cancer. Different biochemical parameters may be utilised for the evaluation of CVD risk in T2DM patients of different age[182]. CRP is an acute-phase protein produces in the liver; its release is stimulated by cytokines (interleukin 6 and tumour necrosis factor alpha). Increased levels of it are related with the presence and severity of CAD and renal impairment in individuals with T2DM[183]. Although the determination of high-sensitivity CRP (hs-CRP) level represents an interest in the screening of CVD in T2DM patients[184].
Increased concentrations of hs-CRP are associated with IR, T2DM and the development of CVD. In particular, inflammation strongly linked with endothelial dysfunction is accepted as one of the cardiovascular risk factors clustering in the IR syndrome or metabolic syndrome. Moreover, low-grade inflammation might play an important role in the pathobiology of the metabolic syndrome[185,186]. The exact mechanism linking IR and inflammation remain unclear. Several studies have drawn attention to the finding of increased levels of hs-CRP in T2DM patients with features of the metabolic syndrome[187-190]. The elevation of hs-CRP was strongly correlated with BMI, serum lipids, fasting glucose and WC[191-197], features of the metabolic syndrome, indicating potential roles of obesity and abdominal obesity in the development of inflammation associated with the metabolic syndrome in T2DM patients. The strong association between IR and inflammation in atherogenesis insinuates that therapies that address both parameters, such thiazolidinediones may have benefits in decreasing diabetes-related macrovascular complications[198].
Serum concentration of hs-CRP is a good biomarker of chronic low-grade inflammation and is an established prognostic marker in acute coronary syndrome. In subjects with DM, the presence of high plasma levels of hs-CRP are predictive for fatal and non-fatal CHD[199,200]. Although suffering from an acute CAD, patients with T2DM have a poor outcome compared with non-diabetic patients, in part explained by a persistent endothelium-dependent dysfunction and inflammatory activity in these patients after acute myocardial infarction[201]. Finally, hs-CRP was related with silent myocardial ischemia, and might help to detect silent myocardial ischemia in diabetic patients[202].
On the other hand, in T2DM patients hs-CRP was an independent risk factor for CHD deaths[203]. In a case control study including 60 T2DM subjects with normal lipid profile and 60 age and sex-matched healthy controls, hs-CRP was an independent cardiac risk predictor even with normal lipid profile and can help measure additional risk[204]. Moreover the Diabetes Heart Study (DHS) documents the utility of hs-CRP in predicting risk for all-cause mortality in 846 European Americans with T2DM, and supports its use as a screening tool in risk prediction models[205]. However, in acute coronary syndrome few studies found no significant differences in hs-CRP between patients with and without diabetes[206]. Khatana et al[207] have found that hs-CRP may not be suitable to predict changes in cardiovascular risk among diabetic patients, and should not be a surrogate for achieving evidence based goals in traditional cardiovascular risk factors; in the Prospective Evaluation of Diabetic Ischaemia Heart Disease by Coronary Tomography Study, there was a negative association between coronary artery calcification score, obtained by electron beam tomography, and CRP in T2DM patients[208]; and the Irbesartan Diabetic Nephropathy Trial with baseline data obtained from 722 diabetic nephropathy patients showed a lack of association between hs-CRP and specific established or emerging cardiovascular risk factors[209].
Diabetic patients with MA and hypertension had more frequent association with increased marker of inflammation such hs-CRP[210]. The correlation found between hs-CRP levels and albuminuria in T2DM patients[211] suggest that the inflammatory process plays a role in diabetic nephropathy patients. However, in these patients CRP does not add predictive information above and beyond that offered by traditional established risk factors[212].
Several large prospective studies have proved that baseline levels of hs-CRP are an independent predictor of cardiovascular events among apparently healthy individuals. However, prospective data on whether hs-CRP predicts cardiovascular events in diabetic patients are limited so far. The Prevention of Renal and Vascular End stage Disease study, a prospective population-based cohort study in the Nederland’s, including 8592 participants[213] show that elevated hs-CRP, has added value to the present metabolic syndrome defining variables in predicting new onset CVD. In a prospective cohort study, baseline hs-CRP level is associated with increased first cardio-cerebral vascular event in the population with DM[214]. In the Casale Monferrato Study[215], hs-CRP measurement is independently related with short-term mortality risk in T2DM patients, even in normoalbuminuric individuals, and in those without a prior diagnosis of CVD; and in the Chennai Urban Rural Epidemiology Study (CURES)[216], an ongoing population-based study conducted on 150 subjects selected from the CURES, hs-CRP demonstrated a solid association with CAD and diabetes even after adjusting for age and gender. Finally, in a prospective study a cohort of 746 American men aged 46-81 years who were free of CVD at the time of blood collection in 1993-1994 were followed[217]. In this study elevated plasma levels of hs-CRP were related with an improved risk of incident cardiovascular events among diabetic men, independent of currently established lifestyle risk factors, blood lipids and glycaemic control.
On the other hand, in the recent ADVANCE study[218], the authors deduce that interleukin-6 levels but not CRP or fibrinogen levels, add significantly to the prediction of macrovascular events and mortality in patients with T2DM who have baseline CVD or risk factors.
In conclusion, the serum levels of hs-CRP, which is a marker of systemic inflammation and a mediator of atherosclerotic disease, have been correlated with the risk of CVD in T2DM patients. The determination of it is very important as screening of CVD in T2DM patients.
Homocysteine (HC) is a sulphur-containing essential amino acid derived from methionine. Vitamins B6, B12 and folic acid act as coenzymes in the metabolism of methionine and HC, and individual deficiency may cause hyperhomocysteinemia (HHC)[219]. Therefore, a negative correlation exists between HC plasma levels and vitamins B6, B12 and folic acid levels[220]. The HC plasma levels are higher in men, in women they increase after the menopause, and in both sexes they rise with aging[219]. An increase in HC levels has also been described in chronic kidney disease through a mechanism that is still not entirely understood, although it has been related to decreased renal clearance and metabolism and/or a descent in the extrarenal metabolism resulting from retained inhibitory substances[221].
In T2DM subjects, elevated HC levels have been related with a rise in the risk of suffering from cardiovascular events, independent of other risk factors[222,223], such as age and renal function[223]. The close relation between HC and CVD confirms the atherosclerotic role in the same[224]. For some authors, higher HC levels are consistent not only with aging and the male gender, but also in line with the development of DM[225]. HC levels do not appear to be related with anthropometric indices such as weight, BMI, percentage of fat mass and triceps skin fold[226].
On the other hand, the HC could play an etiologic role in the pathogenesis of T2DM, promoting OE, systemic inflammation and endothelial dysfunction[227]. HC seems to be the cause of increased mortality in T2DM subjects[223], and some authors consider it as a predictor of mortality[228]. The highest HC levels have been found in diabetic patients who have suffered several cardiovascular events[222].
The role of HC as a cardiovascular risk factor in DM is unclear. The poor metabolic control of the T2DM patients appears to have a predominant role. There exists a positive correlation between the HC levels and those of HbA1c, and a negative correlation with those of insulin[229]. On the other hand, a decline in HC levels has been observed in diabetic patients with a high cardiovascular risk and an elevated intake of foods high in folate, and vitamins B6 and B12[230]. Furthermore, an important predictor of cardiovascular risk in T2DM is arterial compliance which may not only be associated with age, but also with HC levels and renal function parameters[231].
The HHC as a cardiovascular risk factor includes CHD, both in the general population and in the diabetic population, although the role it plays on T2DM is unknown. However, the HHC in plasma is closely related to the development of CAD[232]. Thus, elevated HC levels have been found in patients with CHD, closely correlated with the occurrence of the same in the presence of decreased levels of folic acid and HDL-C[233].
Silent myocardial ischemia is one of the most frequent causes of mortality in the United States and it not only affects the diabetic population. Traditional risk factors have been identified such as T2DM itself, hypertension, dyslipidaemia and smoking, but there are also a series of novel factors such as lipoprotein (a), CRP and HC that can help improve the evaluation of patients with this disease[233]. In patients with T2DM, silent myocardial infarctions have been associated with these novel cardiovascular risk factors such as increased HC[234]. The HHC is related with increased mortality in T2DM patients suffering from CAD, without, however, being a predictor factor of cardiovascular mortality[235].
MA is a predictor of CVD and shows a close relationship with HC. The reason for this association is unknown; however it could be in the origin of MA. There are studies that show a relationship between HC and MA, irrespective of T2DM and hypertension[236]. In T2DM subjects with a high prevalence of peripheral arterial disease and nephropathy, there exists a relationship between the levels of HC and those of MA[226]. Finally, HHC is considered as a risk factor for the development of peripheral arterial disease in T2DM individuals over 65 years of age[237].
HHC is linked with the risk of developing peripheral and autonomic neuropathy. In T2DM, HC is associated with neuropathy developing through an ischemia. The rise of HC appears to be independently associated with autonomic neuropathy, showing no association with peripheral diabetic neuropathy. For each increase in HC, there is a 7.1% increased risk of developing autonomic neuropathy[238].
In a study of T2DM subjects vs non diabetic control subjects, HC levels were found as elevated as those found with preproliferative retinopathy and glaucoma, suggesting that HC was a risk factor for the development of microvascular lesions in these subjects[239]. Small HC elevations in patients with diabetic retinopathy have been associated with capillary and endothelial dysregulation, in which the HHC could be an important risk factor for the development of a macular oedema[240].
An increase in HC levels has been described together with a decrease in levels of folic acid and vitamin B12[241]. However, taking folic acid, and vitamins B12 and B6 supplements with the aim of reducing HC levels does not decrease the risk of developing CVD[237]. Vitamin B12 deficiency together with an elevation of HC will predispose towards an augmented risk of cardiovascular morbidity and mortality in T2DM subjects. Vitamin B12 supplementation in these patients will not reduce the cardiovascular risk[242].
As regards vitamin A, it is capable of affecting the inflammatory mechanisms and the immune function and therefore be associated to CVD. However, there does not appear to be a relation to the cardiovascular risk, as variations of the same are not found in T2DM[243,244]. Neither has an association been found between the zinc levels and cardiovascular risk with the HbA1c levels in T2DM patients[244].
The actions of vitamin D are mediated by binding to a specific nuclear vitamin D receptor (VDR)[247]. Allelic variations of the VDR gene are related with improved risk of CAD in T2DM patients[245]. The hypothesis that vitamin D might protect against vascular disease, comprising atherosclerosis and endothelial dysfunction, is postulated since it has been observed that the VDR is also expressed in the vasculature[246]. An increased production of NO, the inhibition of macrophage to foam cell formation, or a decreased expression of adhesion molecules in endothelial cells, might mediate the vascular protective actions of vitamin D[247-249]. Both endothelial dysfunction and increased arterial stiffness[246,250], and more recently cardiovascular risk factors including T2DM[251], and an elevated risk of CVD[252] are related with low vitamin D levels. Of published observational studies, most have shown that lower levels of vitamin D are related with a high incidence of cardiovascular events and mortality[253-257]. Even asymptomatic CAD was associated with lower vitamin D levels in high risk T2DM patients, as observed in a recent observational study[258]. On the other hand, in T2DM patients, severe vitamin D deficiency predicts improved risk of all-cause and cardiovascular mortality, independent of urinary albumin excretion rate and conventional cardiovascular risk factors[259], and vitamin D deficiency appears to be a significant risk factor for T2DM severity and associated cardio-metabolic risk[260]. Furthermore, in a double-blind, parallel group, placebo-controlled randomized trial, a single large dose of 100000 IU vitamin D2 improves endothelial function in patients with T2DM and vitamin D insufficiency[261]; and in a prospective study, vitamin D supplementation (2000 IU/d) in patients with T2DM on different therapeutic regimens, those patients on insulin in combination with other drugs was the group that benefited the most as compared with other groups in terms of improving cardiovascular risk[262]. Thus, we can conclude that in T2DM vitamin D deficiency is an independent cardiovascular risk factor, but whether vitamin D supplementation can significantly improve cardiovascular outcomes is yet largely unknown. However, early intervention may be considered to improve prevention of T2DM related cardiovascular complications.
It has been believed for years that caffeine, one of the substances most used worldwide and included in coffee, tea, energy drinks and chocolate, increases coronary risk, hypertension and HC concentrations. However their high consumption could modulate insulin sensitivity and blood glucose levels, and in the long term it may reduce the incidence of T2DM[263]. Therefore caffeine would not have any adverse cardiovascular effects, as it demonstrates an antioxidant capacity, and presents an inverse risk association with regard to T2DM[264].
Chronic alcoholism may produce an HC plasma increase due to nutritional deficiencies associated with the said habit[265,266]. An association between alcohol and the development of atherosclerosis has been observed in patients with T2DM. Alcohol consumption and HHC together could explain the occurrence of atherosclerosis in diabetic subjects[267].
Finally, regarding treatments for T2DM, metformin appears to reduce folic acid levels in the blood, which in the long-term would raise HC levels. Folate management in these patients would reduce the levels of the same[268].
Men with DM have a higher prevalence of erectile dysfunction (ED) compared with the general population[269]. In these individuals, the prevalence of ED augments with age and duration and severity of disease[269,270]. ED and atherosclerosis are frequent complications of DM[271]. There are close relations between ED and atherosclerosis in patients with T2DM, and ED might serve as a clinical marker for coronary, peripheral, or cerebrovascular diseases in these subjects[272]. Several studies have found a positive correlation between ED and the risk of cardiovascular events[273,274]. The total cardiovascular risk increases severity of ED in T2DM patients without having overt CVD[275]. A cohort study concluded that the presence of ED in men with T2DM and without clinically overt CVD predicted CHD[276], and another study indicates that ED appears to be robustly and independently related with silent CAD in apparently uncomplicated T2DM subjects[272]. Moreover, a meta-analysis of observational studies concludes that the presence of ED was related with an elevated risk of cardiovascular events in diabetic men[277].
Finally, a recent paper suggests that vitamin D deficiency is closely related with both ED and CVD, and the authors postulate that optimizing serum vitamin D levels through vitamin D supplementation helps delay the onset of ED[278].
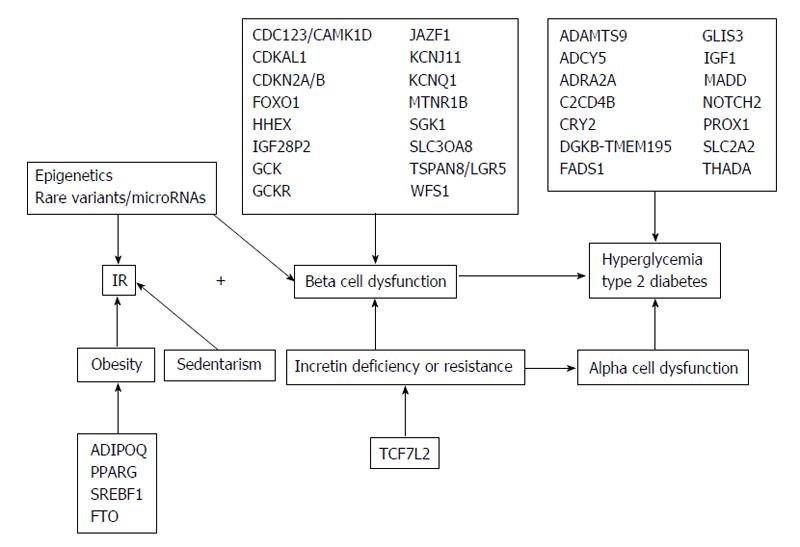
T2DM is an independent risk factor for developing CVD with the relative risk of CVD mortality of 4.9 in women and 2.1 in men, relative to non-diabetics subjects[165,279]. Genetic and environmental factors contribute to this risk. In the past decade, genome-wide association studies had elevated the number of common single-nucleotide polymorphisms, which confirmed the relationship between T2DM and CVD (Figure 3).
Haptoglobin polymorphisms and CVD in T2DM: Haptoglobin (HP) has been involved in both T2DM, and T2DM related CVD[280,281]. HP binds to ApoA1 in the same location as lecithin-cholesterol acyltransferase (LCAT); this lead to a decrease LCAT activity and consequently limiting HDL maturation. This inhibits reverse cholesterol transport causing HDL to become proatherogenic[282].
Several studies have investigated HP polymorphisms and CVD risk in T2DM. In 2002 Levy et al[283] reported an OR of CVD events in diabetes five times greater with the HP 2-2 phenotype, than with HP 1-1 in a study that involved 206 CVD patients and 206 CVD controls (146 and 93 were affected by T2DM, respectively, as part of the Strong Heart Study). In 2004, a subsequent study by Levy et al[284] involved 3273 subjects in the Framingham Heart Study, however only a subset of 433 patients were affected with T2DM, and of these, only 86 had a history of prevalent CVD. Finally, a 2003 study in patients with acute myocardial infarction reported that individuals with T2DM and the HP2 allele had improved mortality following acute myocardial infarction, compared to subjects with T2DM and the HP 1-1 genotype (included only 224 T2DM affected patients)[285]. The DHS is a study of the genetic and epidemiological causes of CVD in patients with T2DM. In a sub-study of 1208 subjects from the DHS, the HP 2-2 genotype was associated with increased carotid intima-media thickness[286].
Apolipoprotein E gene polymorphism and risk of CVD in T2DM: ApoE plays an important role in lipid metabolism as a ligand for many cell-surface receptors comprising the LDL receptor, LDL-receptor related protein and VLDL receptor[287]. Human ApoE is genetically controlled by three alleles (e2, e3, and e4) at a single gene locus in chromosome 19; these code for three isoforms (E2, E3, and E4) and thus determine the six genotypes (e2/2, e4/2, e3/2, e3/3, e4/3, and e4/4)[287]. ApoE ε2 allele has been reported to be related with higher plasma levels of ApoE, reduced plasma levels of LDL-C and lower risk of CAD[288], while ApoE ε4 is related with lower plasma level of ApoE, elevated plasma levels of total cholesterol, LDL-C, VLDL-C, and greater risk of CAD when compared to ApoE3 homozygotes[289]. In diabetic population, Apoe4 allele is related with the risk of CAD[290,291], augmented occurrence of exercise-induced silent myocardial ischemia[292], impairment of endothelium-dependent artery dilation[293], and CAD death[294].
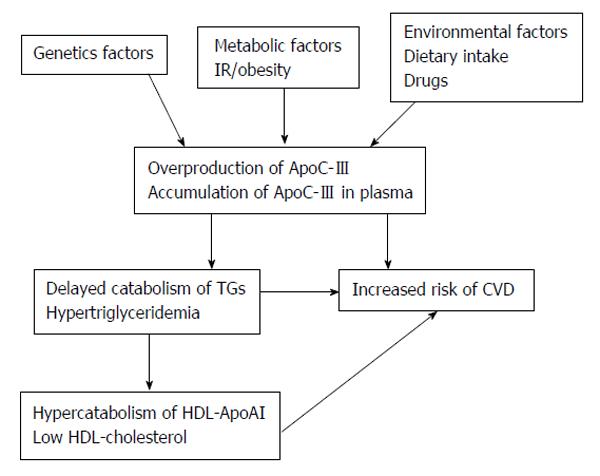
Genetic factors in the overproduction of Apolipoprotein C-III and the risk of CVD in T2DM: Apolipoprotein C-III (ApoC-III) plays an important role in regulating the metabolism of TGs-rich lipoproteins (TRLs). ApoC-III is an inhibitor of lipoprotein lipase and of TRLs remnant uptake by hepatic lipoprotein receptors. Elevated ApoC-III, may cause accumulation of plasma TRLs lead to hypertriglyceridaemia (Figure 4).
The APOC3 gene exists in a gene cluster with the ApoAIV and ApoAI genes on chromosome 11q23[295]. ApoC-III expression is down-regulated, in part, by insulin via the promoter insulin response element on the APOC3 gene[296]. This indicates that ApoC-III expression can be regulated by insulin sensitivity[297]. IR may blunt the sensitivity to the normal insulin-mediated suppression of ApoC-III gene expression. The transcription of APOC3 gene is also mediated by peroxisome proliferator activated receptors (PPAR)[298]. The induction of PPAR, principally the PPAR-α form, decreases APOC3 gene expression[299,300].
Several studies reveal that naturally occurring sequence variation in APOC3 genes affects plasma ApoC-III (and TGs) concentrations in humans. The APOC3 promoter variants at positions -455 and -482 have been studied more extensively because they relate to responsiveness to insulin. Moreover, there is increasing evidence showing the possibility of interactive effects between the APOC3 gene variant and other environmental factors such as dietary intakes or smoking[301,302].
Epigenetics and the risk of CVD in T2DM: There is evidence linking epigenetic factors to various diseases such as DM and CVD[303]. Epigenetic factors could be an important mediator between DM, CVD and chronic inflammatory response, and, by different types of reactions such as acetylation and methylation, could mediate the interaction between genes and environment resulting in activation, repression or silencing the genetic transcription (Figure 5). In particular, DNA methylation has been linked to several cardiovascular-related biomarkers, including HC and CRP[304]. Hyperglycaemia may induce epigenetic changes of genes involved in vascular inflammation. Poor glycemic control increases nuclear transcription factor-κB (NF-κB), which regulates the expression of genes involved in inflammatory diseases, including atherosclerosis and diabetic complications[305], activity in monocytes and gene expression of inflammatory cytokines[306,307]. Moreover, in human aortic endothelial cells, the excess of reactive oxygen species resulting from a transient exposure to hyperglycaemia (16 h) can induce monomethylation of lysine from histone 3, increasing the expression of the subunit p65 of NF-κB[308], responsible for the increased transcription of VCAM-1, monocyte chemoattractant molecule 1, and some inflammatory proteins like interleukin-6, ICAM-1, and NOS, that are associated to hyperglycaemia-induced arterial pathology[307]. Epigenetic changes in the NF-κB p65 promoter induced by transient hyperglycaemia, which persist for 6 d during culture at normal glucose levels, can be regulate for two histones: a histone methylase and a histone demethylase[309]. In fact, the hyperglycaemic memory could be explained by epigenetic changes induced by transient hyperglycemia. Epidemiological studies insinuate that hyperglycemia may induce epigenetic changes of proinflammatory genes, which subsequently regulate gene expression and thereby the development of vascular inflammation[310].
The complex interaction of risk factors in T2DM make it necessary to apply a holistic approach to the management of this chronic disorder, and a comprehensive care plan should therefore include modification of all cardiovascular risk factors. Targeting multiple markers of CVD risk hopefully offers the best chance of improving CVD outcomes.
There are consistent evidences that optimal glycaemic control, along with control of hypertension, dyslipidaemia, smoking cessation, and weight loss are necessary for reducing cardiovascular risk in T2DM patients. Cardiovascular benefits are obtained if the control of traditional cardiovascular risk factors begins early in subjects with short duration of DM and low cardiovascular risk. On the contrary, in elderly subjects with long duration of DM, exposed to hyperglycemia for a long time, and high cardiovascular risk, the same is not true. This beneficial or harmful effect could be explained by the hypothesis called as metabolic memory, in which the effect of the early glycemic exposure environment is imprinted in target organs, resulting in long-term protective or deleterious long-term effects.
In recent years there have been major advances of the influence of non-traditional risk factors for CVD in DM. This knowledge should gradually lead to the development of more effective therapeutic strategies to prevent cardiovascular events. Currently there is no evidence that routine monitoring of these risk factors in diabetic patients leads to better diagnostic and therapeutic results. Nor is there solid evidence to justify screening for subclinical atherosclerosis in asymptomatic subjects with DM.
Further work is needed to understand the impact of epigenetic changes of complications of T2DM, which can lead to the development of new therapeutic strategies for these patients. Research should focus on the factors that lead to dysfunction and failure of islet, particularly those acquired at an early age because they can be prevented. Epigenetic regulation of metabolic genes may be one of the fields of research.
P- Reviewer: Collino M, Okumura K, Ray S, Yin JY S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Harris M, Zimmet P. Classification of diabetes mellitus and other categories of glucose intolerance. International Textbook of Diabetes Mellitus. Chichester: John Willey and Sons Ltd 1997; 9-23. [Cited in This Article: ] |
| 2. | International Diabetes Federation 2011. The Global Burden Prevalence and Projections 2011 and 2030. Available from: http: //www.idf.org/diabetesatlas/5e/the-global-burden. [Cited in This Article: ] |
| 3. | Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in US adults. JAMA. 1999;281:1291-1297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 582] [Cited by in F6Publishing: 538] [Article Influence: 21.5] [Reference Citation Analysis (2)] |
| 4. | Prevalence of small vessel and large vessel disease in diabetic patients from 14 centres. The World Health Organisation Multinational Study of Vascular Disease in Diabetics. Diabetes Drafting Group. Diabetologia. 1985;28 Suppl:615-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 232] [Article Influence: 5.9] [Reference Citation Analysis (3)] |
| 5. | Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4846] [Cited by in F6Publishing: 4384] [Article Influence: 168.6] [Reference Citation Analysis (1)] |
| 6. | Evans JM, Wang J, Morris AD. Comparison of cardiovascular risk between patients with type 2 diabetes and those who had had a myocardial infarction: cross sectional and cohort studies. BMJ. 2002;324:939-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 289] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 7. | Echouffo-Tcheugui JB, Kengne AP. On the importance of global cardiovascular risk assessment in people with type 2 diabetes. Prim Care Diabetes. 2013;7:95-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3042] [Cited by in F6Publishing: 2784] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 9. | del Cañizo Gómez FJ, Moreira Andrés MN. Strict control of modifiable cardiovascular risk factors in patients with type 2 diabetes mellitus. Med Clin (Barc). 2008;130:641-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | del Cañizo-Gómez FJ, Moreira-Andrés MN. Cardiovascular risk factors in patients with type 2 diabetes. Do we follow the guidelines? Diabetes Res Clin Pract. 2004;65:125-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (1)] |
| 11. | Fonseca VA. Risk factors for coronary heart disease in diabetes. Ann Intern Med. 2000;133:154-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Saito I, Folsom AR, Brancati FL, Duncan BB, Chambless LE, McGovern PG. Nontraditional risk factors for coronary heart disease incidence among persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Ann Intern Med. 2000;133:81-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 140] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Jialal I, Stein D, Balis D, Grundy SM, Adams-Huet B, Devaraj S. Effect of hydroxymethyl glutaryl coenzyme a reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation. 2001;103:1933-1935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 481] [Cited by in F6Publishing: 507] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 14. | Parulkar AA, Pendergrass ML, Granda-Ayala R, Lee TR, Fonseca VA. Nonhypoglycemic effects of thiazolidinediones. Ann Intern Med. 2001;134:61-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 323] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 15. | Adler AI, Stevens RJ, Neil A, Stratton IM, Boulton AJ, Holman RR. UKPDS 59: hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes Care. 2002;25:894-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 224] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Ahmed I, Goldstein BJ. Cardiovascular risk in the spectrum of type 2 diabetes mellitus. Mt Sinai J Med. 2006;73:759-768. [PubMed] [Cited in This Article: ] |
| 17. | Jialal I, Devaraj S, Venugopal SK. Oxidative stress, inflammation, and diabetic vasculopathies: the role of alpha tocopherol therapy. Free Radic Res. 2002;36:1331-1336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6145] [Cited by in F6Publishing: 5931] [Article Influence: 257.9] [Reference Citation Analysis (0)] |
| 19. | Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785-1792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2021] [Cited by in F6Publishing: 1996] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 20. | Devaraj S, Jialal I. Oxidized low-density lipoprotein and atherosclerosis. Int J Clin Lab Res. 1996;26:178-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Ceriello A, Mercuri F, Quagliaro L, Assaloni R, Motz E, Tonutti L, Taboga C. Detection of nitrotyrosine in the diabetic plasma: evidence of oxidative stress. Diabetologia. 2001;44:834-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 317] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Gopaul NK, Anggård EE, Mallet AI, Betteridge DJ, Wolff SP, Nourooz-Zadeh J. Plasma 8-epi-PGF2 alpha levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS Lett. 1995;368:225-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 311] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 23. | Davì G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, Santarone S, Pennese E, Vitacolonna E, Bucciarelli T, Costantini F. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99:224-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 531] [Cited by in F6Publishing: 550] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 24. | Dandona P, Thusu K, Cook S, Snyder B, Makowski J, Armstrong D, Nicotera T. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996;347:444-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 564] [Cited by in F6Publishing: 541] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 25. | Fonseca V, Desouza C, Asnani S, Jialal I. Nontraditional risk factors for cardiovascular disease in diabetes. Endocr Rev. 2004;25:153-175. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen MR, Tendera M, Tuomilehto J, Valensi P, Zamorano JL; ESC Committee for Practice Guidelines (CPG), Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document Reviewers, De Backer G, Sirnes PA, Ezquerra EA, Avogaro A, Badimon L, Baranova E, Baumgartner H, Betteridge J, Ceriello A, Fagard R, Funck-Brentano C, Gulba DC, Hasdai D, Hoes AW, Kjekshus JK, Knuuti J, Kolh P, Lev E, Mueller C, Neyses L, Nilsson PM, Perk J, Ponikowski P, Reiner Z, Sattar N, Schächinger V, Scheen A, Schirmer H, Strömberg A, Sudzhaeva S, Tamargo JL, Viigimaa M, Vlachopoulos C, Xuereb RG. L. The Task Force on diabetes, pré-diabetes and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). ESC Guidelines on diabetes, pre-diabetes and cardiovascular diseases, developed in collaboration with the EASD. Eur Heart J. 2013;34:3035-3087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1378] [Cited by in F6Publishing: 1394] [Article Influence: 126.7] [Reference Citation Analysis (0)] |
| 27. | Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986;314:488-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3689] [Cited by in F6Publishing: 3393] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 28. | Stern MP. Diabetes and cardiovascular disease. The “common soil” hypothesis. Diabetes. 1995;44:369-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 153] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Cannon CP. Mixed dyslipidemia, metabolic syndrome, diabetes mellitus, and cardiovascular disease: clinical implications. Am J Cardiol. 2008;102:5L-9L. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 52] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 30. | Sorrentino SA, Besler C, Rohrer L, Meyer M, Heinrich K, Bahlmann FH, Mueller M, Horváth T, Doerries C, Heinemann M. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation. 2010;121:110-122. [PubMed] [Cited in This Article: ] |
| 31. | Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Borén J, Catapano AL, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32:1345-1361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 32. | Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292-2333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (1)] |
| 33. | Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, Kastelein JJ, Bittner V, Fruchart JC. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1141] [Cited by in F6Publishing: 1101] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 34. | Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670-1681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4136] [Cited by in F6Publishing: 4248] [Article Influence: 303.4] [Reference Citation Analysis (1)] |
| 35. | Scott R, O’Brien R, Fulcher G, Pardy C, D’Emden M, Tse D, Taskinen MR, Ehnholm C, Keech A. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32:493-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 433] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 36. | Ginsberg HN, Elam MB, Lovato LC, Crouse JR, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1929] [Cited by in F6Publishing: 1818] [Article Influence: 129.9] [Reference Citation Analysis (0)] |
| 37. | Taskinen MR, Barter PJ, Ehnholm C, Sullivan DR, Mann K, Simes J, Best JD, Hamwood S, Keech AC. Ability of traditional lipid ratios and apolipoprotein ratios to predict cardiovascular risk in people with type 2 diabetes. Diabetologia. 2010;53:1846-1855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993-2000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1866] [Cited by in F6Publishing: 1971] [Article Influence: 131.4] [Reference Citation Analysis (0)] |
| 39. | Mills EJ, O’Regan C, Eyawo O, Wu P, Mills F, Berwanger O, Briel M. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of > 40 000 patients. Eur Heart J. 2011;32:1409-1415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2728] [Cited by in F6Publishing: 2547] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 41. | Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005-2016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1734] [Cited by in F6Publishing: 1755] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 42. | Sever PS, Poulter NR, Dahlöf B, Wedel H, Collins R, Beevers G, Caulfield M, Kjeldsen SE, Kristinsson A, McInnes GT. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial--lipid-lowering arm (ASCOT-LLA). Diabetes Care. 2005;28:1151-1157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 259] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 43. | Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849-1861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2338] [Cited by in F6Publishing: 2129] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 44. | Bruckert E, Labreuche J, Deplanque D, Touboul PJ, Amarenco P. Fibrates effect on cardiovascular risk is greater in patients with high triglyceride levels or atherogenic dyslipidemia profile: a systematic review and meta-analysis. J Cardiovasc Pharmacol. 2011;57:267-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 45. | Jun M, Foote C, Lv J, Neal B, Patel A, Nicholls SJ, Grobbee DE, Cass A, Chalmers J, Perkovic V. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet. 2010;375:1875-1884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 648] [Cited by in F6Publishing: 608] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 46. | Nilsson PM, Cederholm J, Zethelius BR, Eliasson BR, Eeg-Olofsson K, Gudbj Rnsdottir S. Trends in blood pressure control in patients with type 2 diabetes: data from the Swedish National Diabetes Register (NDR). Blood Press. 2011;20:348-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Redon J, Cifkova R, Laurent S, Nilsson P, Narkiewicz K, Erdine S, Mancia G. Mechanisms of hypertension in the cardiometabolic syndrome. J Hypertens. 2009;27:441-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Mogensen CE. New treatment guidelines for a patient with diabetes and hypertension. J Hypertens Suppl. 2003;21:S25-S30. [PubMed] [Cited in This Article: ] |
| 49. | Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4930] [Cited by in F6Publishing: 4180] [Article Influence: 160.8] [Reference Citation Analysis (0)] |
| 50. | Cushman WC, Evans GW, Byington RP, Goff DC, Grimm RH, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2461] [Cited by in F6Publishing: 2309] [Article Influence: 164.9] [Reference Citation Analysis (0)] |
| 51. | Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1475] [Cited by in F6Publishing: 1379] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 52. | Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008;359:1565-1576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 549] [Cited by in F6Publishing: 617] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 53. | Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, Wright AD, Turner RC, Holman RR. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1386] [Cited by in F6Publishing: 1259] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 54. | Hata J, Arima H, Rothwell PM, Woodward M, Zoungas S, Anderson C, Patel A, Neal B, Glasziou P, Hamet P. Effects of visit-to-visit variability in systolic blood pressure on macrovascular and microvascular complications in patients with type 2 diabetes mellitus: the ADVANCE trial. Circulation. 2013;128:1325-1334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 55. | Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C, Zampi I, Santucci A. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1171] [Cited by in F6Publishing: 1112] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 56. | Pringle E, Phillips C, Thijs L, Davidson C, Staessen JA, de Leeuw PW, Jaaskivi M, Nachev C, Parati G, O’Brien ET. Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens. 2003;21:2251-2257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 276] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 57. | Tamura K, Tsurumi Y, Sakai M, Tanaka Y, Okano Y, Yamauchi J, Ishigami T, Kihara M, Hirawa N, Toya Y. A possible relationship of nocturnal blood pressure variability with coronary artery disease in diabetic nephropathy. Clin Exp Hypertens. 2007;29:31-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Pierdomenico SD, Di Nicola M, Esposito AL, Di Mascio R, Ballone E, Lapenna D, Cuccurullo F. Prognostic value of different indices of blood pressure variability in hypertensive patients. Am J Hypertens. 2009;22:842-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 59. | Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1236] [Cited by in F6Publishing: 1220] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 60. | Kikuya M, Ohkubo T, Metoki H, Asayama K, Hara A, Obara T, Inoue R, Hoshi H, Hashimoto J, Totsune K. Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: the Ohasama study. Hypertension. 2008;52:1045-1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 313] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 61. | Hsieh YT, Tu ST, Cho TJ, Chang SJ, Chen JF, Hsieh MC. Visit-to-visit variability in blood pressure strongly predicts all-cause mortality in patients with type 2 diabetes: a 5·5-year prospective analysis. Eur J Clin Invest. 2012;42:245-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 62. | Hata Y, Kimura Y, Muratani H, Fukiyama K, Kawano Y, Ashida T, Yokouchi M, Imai Y, Ozawa T, Fujii J. Office blood pressure variability as a predictor of brain infarction in elderly hypertensive patients. Hypertens Res. 2000;23:553-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 63. | Hata Y, Muratani H, Kimura Y, Fukiyama K, Kawano Y, Ashida T, Yokouchi M, Imai Y, Ozawa T, Fujii J. Office blood pressure variability as a predictor of acute myocardial infarction in elderly patients receiving antihypertensive therapy. J Hum Hypertens. 2002;16:141-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 64. | Franklin SS, Thijs L, Li Y, Hansen TW, Boggia J, Liu Y, Asayama K, Björklund-Bodegård K, Ohkubo T, Jeppesen J. Masked hypertension in diabetes mellitus: treatment implications for clinical practice. Hypertension. 2013;61:964-971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 65. | Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, Li Y, Dolan E, Tikhonoff V, Seidlerová J, Kuznetsova T, Stolarz K. The International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood Press Monit. 2007;12:255-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 66. | Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6 Suppl 2:51S-209S. [PubMed] [Cited in This Article: ] |
| 67. | Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735-2752. [PubMed] [Cited in This Article: ] |
| 68. | Sobel BE. Optimizing cardiovascular outcomes in diabetes mellitus. Am J Med. 2007;120:S3-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 69. | Sobel BE. Ancillary studies in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial: Synergies and opportunities. Am J Cardiol. 2006;97:53G-58G. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 70. | Eckel RH. Mechanisms of the components of the metabolic syndrome that predispose to diabetes and atherosclerotic CVD. Proc Nutr Soc. 2007;66:82-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1828] [Cited by in F6Publishing: 1866] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 72. | Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2823] [Cited by in F6Publishing: 2914] [Article Influence: 171.4] [Reference Citation Analysis (0)] |
| 73. | Albu JB, Lu J, Mooradian AD, Krone RJ, Nesto RW, Porter MH, Rana JS, Rogers WJ, Sobel BE, Gottlieb SH. Relationships of obesity and fat distribution with atherothrombotic risk factors: baseline results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Obesity (Silver Spring). 2010;18:1046-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Zoppini G, Verlato G, Leuzinger C, Zamboni C, Brun E, Bonora E, Muggeo M. Body mass index and the risk of mortality in type II diabetic patients from Verona. Int J Obes Relat Metab Disord. 2003;27:281-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Czernichow S, Kengne AP, Huxley RR, Batty GD, de Galan B, Grobbee D, Pillai A, Zoungas S, Marre M, Woodward M. Comparison of waist-to-hip ratio and other obesity indices as predictors of cardiovascular disease risk in people with type-2 diabetes: a prospective cohort study from ADVANCE. Eur J Cardiovasc Prev Rehabil. 2011;18:312-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 76. | Powell KE, Thompson PD, Caspersen CJ, Kendrick JS. Physical activity and the incidence of coronary heart disease. Annu Rev Public Health. 1987;8:253-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 895] [Cited by in F6Publishing: 816] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 77. | Shephard RJ, Balady GJ. Exercise as cardiovascular therapy. Circulation. 1999;99:963-972. [PubMed] [Cited in This Article: ] |
| 78. | Gregg EW, Gerzoff RB, Caspersen CJ, Williamson DF, Narayan KM. Relationship of walking to mortality among US adults with diabetes. Arch Intern Med. 2003;163:1440-1447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 362] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 79. | Hu G, Eriksson J, Barengo NC, Lakka TA, Valle TT, Nissinen A, Jousilahti P, Tuomilehto J. Occupational, commuting, and leisure-time physical activity in relation to total and cardiovascular mortality among Finnish subjects with type 2 diabetes. Circulation. 2004;110:666-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 80. | Fang ZY, Sharman J, Prins JB, Marwick TH. Determinants of exercise capacity in patients with type 2 diabetes. Diabetes Care. 2005;28:1643-1648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 81. | Phielix E, Meex R, Moonen-Kornips E, Hesselink MK, Schrauwen P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia. 2010;53:1714-1721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 82. | Winnick JJ, Sherman WM, Habash DL, Stout MB, Failla ML, Belury MA, Schuster DP. Short-term aerobic exercise training in obese humans with type 2 diabetes mellitus improves whole-body insulin sensitivity through gains in peripheral, not hepatic insulin sensitivity. J Clin Endocrinol Metab. 2008;93:771-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 83. | Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297:E151-E156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 84. | Richards JC, Johnson TK, Kuzma JN, Lonac MC, Schweder MM, Voyles WF, Bell C. Short-term sprint interval training increases insulin sensitivity in healthy adults but does not affect the thermogenic response to beta-adrenergic stimulation. J Physiol. 2010;588:2961-2972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 85. | Little JP, Gillen JB, Percival ME, Safdar A, Tarnopolsky MA, Punthakee Z, Jung ME, Gibala MJ. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol (1985). 2011;111:1554-1560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 469] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 86. | Womack L, Peters D, Barrett EJ, Kaul S, Price W, Lindner JR. Abnormal skeletal muscle capillary recruitment during exercise in patients with type 2 diabetes mellitus and microvascular complications. J Am Coll Cardiol. 2009;53:2175-2183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 87. | Chudyk A, Petrella RJ. Effects of exercise on cardiovascular risk factors in type 2 diabetes: a meta-analysis. Diabetes Care. 2011;34:1228-1237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 88. | Sluik D, Buijsse B, Muckelbauer R, Kaaks R, Teucher B, Johnsen NF, Tjønneland A, Overvad K, Ostergaard JN, Amiano P. Physical Activity and Mortality in Individuals With Diabetes Mellitus: A Prospective Study and Meta-analysis. Arch Intern Med. 2012;172:1285-1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 89. | Hu FB, Stampfer MJ, Solomon C, Liu S, Colditz GA, Speizer FE, Willett WC, Manson JE. Physical activity and risk for cardiovascular events in diabetic women. Ann Intern Med. 2001;134:96-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 288] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 90. | Madsbad S, McNair P, Christensen MS, Christiansen C, Faber OK, Binder C, Transbøl I. Influence of smoking on insulin requirement and metbolic status in diabetes mellitus. Diabetes Care. 1980;3:41-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Bott U, Jörgens V, Grüsser M, Bender R, Mühlhauser I, Berger M. Predictors of glycaemic control in type 1 diabetic patients after participation in an intensified treatment and teaching programme. Diabet Med. 1994;11:362-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 92. | Chase HP, Garg SK, Marshall G, Berg CL, Harris S, Jackson WE, Hamman RE. Cigarette smoking increases the risk of albuminuria among subjects with type I diabetes. JAMA. 1991;265:614-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 105] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 93. | Morrish NJ, Stevens LK, Fuller JH, Jarrett RJ, Keen H. Risk factors for macrovascular disease in diabetes mellitus: the London follow-up to the WHO Multinational Study of Vascular Disease in Diabetics. Diabetologia. 1991;34:590-594. [PubMed] [Cited in This Article: ] |
| 94. | Cryer PE, Haymond MW, Santiago JV, Shah SD. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N Engl J Med. 1976;295:573-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 788] [Cited by in F6Publishing: 741] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 95. | Kershbaum A, Bellet S. Smoking as a factor in atherosclerosis. A review of epidemiological, pathological, and experimental studies. Geriatrics. 1966;21:155-170. [PubMed] [Cited in This Article: ] |
| 96. | Kirschbaum C, Wüst S, Strasburger CJ. ‘Normal’ cigarette smoking increases free cortisol in habitual smokers. Life Sci. 1992;50:435-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 124] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 97. | Wilkins JN, Carlson HE, Van Vunakis H, Hill MA, Gritz E, Jarvik ME. Nicotine from cigarette smoking increases circulating levels of cortisol, growth hormone, and prolactin in male chronic smokers. Psychopharmacology (Berl). 1982;78:305-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 174] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 98. | Lucini D, Bertocchi F, Malliani A, Pagani M. A controlled study of the autonomic changes produced by habitual cigarette smoking in healthy subjects. Cardiovasc Res. 1996;31:633-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 99. | Niedermaier ON, Smith ML, Beightol LA, Zukowska-Grojec Z, Goldstein DS, Eckberg DL. Influence of cigarette smoking on human autonomic function. Circulation. 1993;88:562-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 100. | Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11:351-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 432] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 101. | Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Holman RR. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ. 1998;316:823-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1410] [Cited by in F6Publishing: 1290] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 102. | Kothari V, Stevens RJ, Adler AI, Stratton IM, Manley SE, Neil HA, Holman RR. UKPDS 60: risk of stroke in type 2 diabetes estimated by the UK Prospective Diabetes Study risk engine. Stroke. 2002;33:1776-1781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 326] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 103. | Al-Delaimy WK, Willett WC, Manson JE, Speizer FE, Hu FB. Smoking and mortality among women with type 2 diabetes: The Nurses’ Health Study cohort. Diabetes Care. 2001;24:2043-2048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 104. | Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care. 1997;20:1266-1272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 105. | Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635-1701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2587] [Cited by in F6Publishing: 2583] [Article Influence: 215.3] [Reference Citation Analysis (0)] |
| 106. | Bhatia LS, Curzen NP, Calder PC, Byrne CD. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J. 2012;33:1190-1200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 316] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 107. | Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356:213-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1382] [Cited by in F6Publishing: 1312] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 108. | Pyöräla K. Relationship of glucose tolerance and plasma insulin to the incidence of coronary heart disease: results from two population studies in Finland. Diabetes Care. 1979;2:131-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 557] [Cited by in F6Publishing: 494] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 109. | Stout RW. Insulin and atheroma. 20-yr perspective. Diabetes Care. 1990;13:631-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 413] [Cited by in F6Publishing: 428] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 110. | Muntoni S, Muntoni S, Draznin B. Effects of chronic hyperinsulinemia in insulin-resistant patients. Curr Diab Rep. 2008;8:233-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Mitsuhashi T, Hibi K, Kosuge M, Morita S, Komura N, Kusama I, Otsuka F, Endo M, Iwahashi N, Okuda J. Relation between hyperinsulinemia and nonculprit plaque characteristics in nondiabetic patients with acute coronary syndromes. JACC Cardiovasc Imaging. 2011;4:392-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 112. | Cheal KL, Abbasi F, Lamendola C, McLaughlin T, Reaven GM, Ford ES. Relationship to insulin resistance of the adult treatment panel III diagnostic criteria for identification of the metabolic syndrome. Diabetes. 2004;53:1195-1200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 223] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 113. | Russell JC, Proctor SD. Small animal models of cardiovascular disease: tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc Pathol. 2006;15:318-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 114. | Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1943] [Cited by in F6Publishing: 1911] [Article Influence: 106.2] [Reference Citation Analysis (3)] |
| 115. | Montagnani M, Golovchenko I, Kim I, Koh GY, Goalstone ML, Mundhekar AN, Johansen M, Kucik DF, Quon MJ, Draznin B. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. J Biol Chem. 2002;277:1794-1799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 249] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 116. | Ridray S. Hyperinsulinemia and smooth muscle cell proliferation. Int J Obes Relat Metab Disord. 1995;19 Suppl 1:S39-S51. [PubMed] [Cited in This Article: ] |
| 117. | Golovchenko I, Goalstone ML, Watson P, Brownlee M, Draznin B. Hyperinsulinemia enhances transcriptional activity of nuclear factor-kappaB induced by angiotensin II, hyperglycemia, and advanced glycosylation end products in vascular smooth muscle cells. Circ Res. 2000;87:746-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 118. | Madonna R, Pandolfi A, Massaro M, Consoli A, De Caterina R. Insulin enhances vascular cell adhesion molecule-1 expression in human cultured endothelial cells through a pro-atherogenic pathway mediated by p38 mitogen-activated protein-kinase. Diabetologia. 2004;47:532-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 119. | Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179). J Biol Chem. 2001;276:30392-30398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 411] [Cited by in F6Publishing: 416] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 120. | Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000;105:311-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 829] [Cited by in F6Publishing: 755] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 121. | Wang CC, Goalstone ML, Draznin B. Molecular mechanisms of insulin resistance that impact cardiovascular biology. Diabetes. 2004;53:2735-2740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 122. | Cavalot F, Petrelli A, Traversa M, Bonomo K, Fiora E, Conti M, Anfossi G, Costa G, Trovati M. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab. 2006;91:813-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 431] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 123. | O’Keefe JH, Gheewala NM, O’Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol. 2008;51:249-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 346] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 124. | Bell DS, O’Keefe JH, Jellinger P. Postprandial dysmetabolism: the missing link between diabetes and cardiovascular events? Endocr Pract. 2008;14:112-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 125. | O’Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol. 2007;100:899-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 386] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 126. | Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681-1687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1689] [Cited by in F6Publishing: 1684] [Article Influence: 93.6] [Reference Citation Analysis (0)] |
| 127. | Ceriello A, Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A, Esposito K, Giugliano D. Effect of postprandial hypertriglyceridemia and hyperglycemia on circulating adhesion molecules and oxidative stress generation and the possible role of simvastatin treatment. Diabetes. 2004;53:701-710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 258] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 128. | International Diabetes Federation. Guidelines for managementof postmeal glucose in diabetes 2011. Available from: http: www.idf.org/2011-guideline-management-postmeal-glucose-diabetes. [Cited in This Article: ] |
| 129. | O’Keefe JH, Abuannadi M, Lavie CJ, Bell DS. Strategies for optimizing glycemic control and cardiovascular prognosis in patients with type 2 diabetes mellitus. Mayo Clin Proc. 2011;86:128-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 130. | Hanefeld M, Fischer S, Julius U, Schulze J, Schwanebeck U, Schmechel H, Ziegelasch HJ, Lindner J. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577-1583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 653] [Cited by in F6Publishing: 596] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 131. | Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, Erqou S, Sattar N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765-1772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1022] [Cited by in F6Publishing: 972] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 132. | Hanefeld M, Cagatay M, Petrowitsch T, Neuser D, Petzinna D, Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J. 2004;25:10-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 460] [Cited by in F6Publishing: 422] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 133. | Chiasson JL, Gomis R, Hanefeld M, Josse RG, Karasik A, Laakso M. The STOP-NIDDM Trial: an international study on the efficacy of an alpha-glucosidase inhibitor to prevent type 2 diabetes in a population with impaired glucose tolerance: rationale, design, and preliminary screening data. Study to Prevent Non-Insulin-Dependent Diabetes Mellitus. Diabetes Care. 1998;21:1720-1725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 138] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 134. | Holman RR, Haffner SM, McMurray JJ, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley BM. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1463-1476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 323] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 135. | Raz I, Wilson PW, Strojek K, Kowalska I, Bozikov V, Gitt AK, Jermendy G, Campaigne BN, Kerr L, Milicevic Z. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care. 2009;32:381-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 280] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 136. | Frontoni S, Di Bartolo P, Avogaro A, Bosi E, Paolisso G, Ceriello A. Glucose variability: An emerging target for the treatment of diabetes mellitus. Diabetes Res Clin Pract. 2013;102:86-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 137. | Rossing P, Hougaard P, Borch-Johnsen K, Parving HH. Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ. 1996;313:779-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 304] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 138. | Monhart V. [Microalbuminuria. From diabetes to cardiovascular risk]. Vnitr Lek. 2011;57:293-298. [PubMed] [Cited in This Article: ] |
| 139. | Udenze IC, Azinge EC, Ebuehi OA, Awolola NA, Adekola OO, Menkiti I, Irurhe NK. The relationship between microalbuminuria, cardiovascular risk factors and disease management in type 2 diabetes. Nig Q J Hosp Med. 2012;22:34-38. [PubMed] [Cited in This Article: ] |
| 140. | Rossi MC, Nicolucci A, Pellegrini F, Comaschi M, Ceriello A, Cucinotta D, Giorda C, Valentini U, Vespasiani G, De Cosmo S. Identifying patients with type 2 diabetes at high risk of microalbuminuria: results of the DEMAND (Developing Education on Microalbuminuria for Awareness of reNal and cardiovascular risk in Diabetes) Study. Nephrol Dial Transplant. 2008;23:1278-1284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 141. | Al-Futaisi A, Al-Zakwani I, Almahrezi A, Al-Hajri R, Al-Hashmi L, Al-Muniri A, Farooqui M. Prevalence and predictors of microalbuminuria in patients with type 2 diabetes mellitus: a cross-sectional observational study in Oman. Diabetes Res Clin Pract. 2006;72:212-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 142. | Ubink-Veltmaat LJ, Bilo HJ, Meyboom-de Jong B. [Microalbuminuria in patients with type 2 diabetes mellitus in general practice]. Ned Tijdschr Geneeskd. 2004;148:2026-2030. [PubMed] [Cited in This Article: ] |
| 143. | Satchell SC, Tooke JE. What is the mechanism of microalbuminuria in diabetes: a role for the glomerular endothelium? Diabetologia. 2008;51:714-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 223] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 144. | Gimeno-Orna JA, Molinero-Herguedas E, Lou-Arnal LM, Boned-Juliani B, Labrador-Fuster T, Guiu-Campos M. [Microalbuminuria accounts for the increased vascular disease risk in diabetic patients with metabolic syndrome]. Rev Esp Cardiol. 2007;60:1202-1205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 145. | Lane JT. Microalbuminuria as a marker of cardiovascular and renal risk in type 2 diabetes mellitus: a temporal perspective. Am J Physiol Renal Physiol. 2004;286:F442-F450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 146. | Weir MR. Microalbuminuria in type 2 diabetics: an important, overlooked cardiovascular risk factor. J Clin Hypertens (Greenwich). 2004;6:134-141; quiz 142-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 147. | Gimeno-Orna JA, Molinero-Herguedas E, Sánchez-Vaño R, Lou-Arnal LM, Boned-Juliani B, Castro-Alonso FJ. Microalbuminuria presents the same vascular risk as overt CVD in type 2 diabetes. Diabetes Res Clin Pract. 2006;74:103-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 148. | Guo L, Cheng Y, Wang X, Pan Q, Li H, Zhang L, Wang Y. Association between microalbuminuria and cardiovascular disease in type 2 diabetes mellitus of the Beijing Han nationality. Acta Diabetol. 2012;49 Suppl 1:S65-S71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 149. | Reinhard H, Wiinberg N, Hansen PR, Kjær A, Petersen CL, Winther K, Parving HH, Rossing P, Jacobsen PK. NT-proBNP levels, atherosclerosis and vascular function in asymptomatic type 2 diabetic patients with microalbuminuria: peripheral reactive hyperaemia index but not NT-proBNP is an independent predictor of coronary atherosclerosis. Cardiovasc Diabetol. 2011;10:71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 150. | Chico A, Tomás A, Novials A. Silent myocardial ischemia is associated with autonomic neuropathy and other cardiovascular risk factors in type 1 and type 2 diabetic subjects, especially in those with microalbuminuria. Endocrine. 2005;27:213-217. [PubMed] [Cited in This Article: ] |
| 151. | Kalaitzidis R, Bakris G. Pathogenesis and treatment of microalbuminuria in patients with diabetes: the road ahead. J Clin Hypertens (Greenwich). 2009;11:636-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 152. | Reinhard H, Nybo M, Hansen PR, Wiinberg N, Kjær A, Petersen CL, Winther K, Parving HH, Rasmussen LM, Rossing P. Osteoprotegerin and coronary artery disease in type 2 diabetic patients with microalbuminuria. Cardiovasc Diabetol. 2011;10:70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 153. | Del Cañizo Gómez FJ, Fernández Pérez C, Moreno Ruiz I, de Gorospe Pérez-Jáuregui C, Silveira Rodríguez B, González Losada T, Segura Galindo A. Microvascular complications and risk factors in patients with type 2 diabetes. Endocrinol Nutr. 2011;58:163-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 154. | Wakabayashi I, Masuda H. Association of D-dimer with microalbuminuria in patients with type 2 diabetes mellitus. J Thromb Thrombolysis. 2009;27:29-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 155. | Mongkolsomlit S, Patumanond J, Tawichasril C, Komoltri C, Rawdaree P. Meta-regression of risk factors for microalbuminuria in type 2 diabetes. Southeast Asian J Trop Med Public Health. 2012;43:455-466. [PubMed] [Cited in This Article: ] |
| 156. | Adetunji OR, Adeleye JO, Agada NO, Salako BL. Microalbuminuria and clinical correlates in black African patients with type 2 diabetes. West Afr J Med. 2005;25:279-283. [PubMed] [Cited in This Article: ] |
| 157. | Cederholm J, Eliasson B, Nilsson PM, Weiss L, Gudbjörnsdottir S. Microalbuminuria and risk factors in type 1 and type 2 diabetic patients. Diabetes Res Clin Pract. 2005;67:258-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 158. | Zandbergen AA, Sijbrands EJ, Lamberts SW, Bootsma AH. Normotensive women with type 2 diabetes and microalbuminuria are at high risk for macrovascular disease. Diabetes Care. 2006;29:1851-1855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 159. | Yokoyama H, Oishi M, Kawai K, Sone H. Reduced GFR and microalbuminuria are independently associated with prevalent cardiovascular disease in Type 2 diabetes: JDDM study 16. Diabet Med. 2008;25:1426-1432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 160. | Zhan WW, Chen YH, Zhang YF, Zhu Y, Lin YY, Ren XP, Li XY, Liu YP. Carotid stiffness and microalbuminuria in patients with type 2 diabetes. Endocrine. 2009;35:409-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 161. | Shin DI, Seung KB, Yoon HE, Hwang BH, Seo SM, Shin SJ, Kim PJ, Chang K, Baek SH. Microalbuminuria is independently associated with arterial stiffness and vascular inflammation but not with carotid intima-media thickness in patients with newly diagnosed type 2 diabetes or essential hypertension. J Korean Med Sci. 2013;28:252-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 162. | Chu CH, Lam HC, Lee JK, Lu CC, Sun CC, Cheng HJ, Wang MC, Chuang MJ. Carotid intima-media thickness in Chinese Type 2 diabetic subjects with or without microalbuminuria. J Endocrinol Invest. 2012;35:254-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 163. | Khan KN, Khan MH, Haque MZ. Correlation between microalbuminuria with complexity of coronary artery disease in diabetic patients. Mymensingh Med J. 2013;22:353-357. [PubMed] [Cited in This Article: ] |
| 164. | Sukhija R, Aronow WS, Kakar P, Garza L, Sachdeva R, Sinha A, Mehta JL. Relation of microalbuminuria and coronary artery disease in patients with and without diabetes mellitus. Am J Cardiol. 2006;98:279-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 165. | Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035-2038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2337] [Cited by in F6Publishing: 2093] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 166. | Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971-1993. Diabetes Care. 1998;21:1138-1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 603] [Cited by in F6Publishing: 635] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 167. | Badimon L, Badimon JJ, Vilahur G, Segalés E, Llorente V. Pathogenesis of the acute coronary syndromes and therapeutic implications. Pathophysiol Haemost Thromb. 2002;32:225-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 168. | Colwell JA. Vascular thrombosis in type II diabetes mellitus. Diabetes. 1993;42:8-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 169. | U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995;44:1249-1258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1087] [Cited by in F6Publishing: 960] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 170. | Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134-1146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1310] [Cited by in F6Publishing: 1270] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 171. | Wood D, De Backer G, Faergeman O, Graham I, Mancia G, Pyörälä K. Prevention of coronary heart disease in clinical practice. Summary of recommendations of the Second Joint Task Force of European and other Societies on Coronary Prevention. J Hypertens. 1998;16:1407-1414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 115] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 172. | Stratmann B, Tschoepe D. Pathobiology and cell interactions of platelets in diabetes. Diab Vasc Dis Res. 2005;2:16-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 173. | Stratmann B, Tschoepe D. Atherogenesis and atherothrombosis--focus on diabetes mellitus. Best Pract Res Clin Endocrinol Metab. 2009;23:291-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 174. | Kwaan HC. Changes in blood coagulation, platelet function, and plasminogen-plasmin system in diabetes. Diabetes. 1992;41 Suppl 2:32-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 175. | Olexa P, Olexová M. [Plasminogen activator inhibitor-1 (PAI-1), ischemic heart disease and diabetes mellitus]. Vnitr Lek. 2003;49:222-226. [PubMed] [Cited in This Article: ] |
| 176. | Fujii S, Goto D, Zaman T, Ishimori N, Watano K, Kaneko T, Okada H, Makiguchi M, Nakagawa T, Kitabatake A. Diminished fibrinolysis and thrombosis: clinical implications for accelerated atherosclerosis. J Atheroscler Thromb. 1998;5:76-81. [PubMed] [Cited in This Article: ] |
| 177. | McBane RD, Hardison RM, Sobel BE. Comparison of plasminogen activator inhibitor-1, tissue type plasminogen activator antigen, fibrinogen, and D-dimer levels in various age decades in patients with type 2 diabetes mellitus and stable coronary artery disease (from the BARI 2D trial). Am J Cardiol. 2010;105:17-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 178. | Stec JJ, Silbershatz H, Tofler GH, Matheney TH, Sutherland P, Lipinska I, Massaro JM, Wilson PF, Muller JE, D’Agostino RB. Association of fibrinogen with cardiovascular risk factors and cardiovascular disease in the Framingham Offspring Population. Circulation. 2000;102:1634-1638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 189] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 179. | Sherman J, Cooper J. Advanced diagnostic procedures for evaluating the visual status of the child. J Am Optom Assoc. 1979;50:1139-1149. [PubMed] [Cited in This Article: ] |
| 180. | Ceriello A, Taboga C, Giacomello R, Falleti E, De Stasio G, Motz E, Lizzio S, Gonano F, Bartoli E. Fibrinogen plasma levels as a marker of thrombin activation in diabetes. Diabetes. 1994;43:430-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 181. | Gosk-Bierska I, Adamiec R, Alexewicz P, Wysokinski WE. Coagulation in diabetic and non-diabetic claudicants. Int Angiol. 2002;21:128-133. [PubMed] [Cited in This Article: ] |
| 182. | Kalofoutis C, Piperi C, Zisaki A, Singh J, Harris F, Phoenix D, Alaveras A, Kalofoutis A. Differences in expression of cardiovascular risk factors among type 2 diabetes mellitus patients of different age. Ann N Y Acad Sci. 2006;1084:166-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 183. | Lu L, Pu LJ, Xu XW, Zhang Q, Zhang RY, Zhang JS, Hu J, Yang ZK, Lu AK, Ding FH. Association of serum levels of glycated albumin, C-reactive protein and tumor necrosis factor-alpha with the severity of coronary artery disease and renal impairment in patients with type 2 diabetes mellitus. Clin Biochem. 2007;40:810-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 184. | El Oudi M, Aouni Z, Ouertani H, Mazigh C, Nsiri B, Zidi B, Machghoul S. [CRP and micro-albumin: new markers of cardiovascular risk in type 2 diabetes]. Ann Cardiol Angeiol (Paris). 2011;60:207-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 185. | Das UN. Metabolic syndrome X: an inflammatory condition? Curr Hypertens Rep. 2004;6:66-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 186. | Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4404] [Cited by in F6Publishing: 4370] [Article Influence: 174.8] [Reference Citation Analysis (0)] |
| 187. | Fröhlich M, Imhof A, Berg G, Hutchinson WL, Pepys MB, Boeing H, Muche R, Brenner H, Koenig W. Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care. 2000;23:1835-1839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 449] [Cited by in F6Publishing: 476] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 188. | Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean ME, Haffner SM. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care. 2002;25:2016-2021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 347] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 189. | Laaksonen DE, Niskanen L, Nyyssönen K, Punnonen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT. C-reactive protein and the development of the metabolic syndrome and diabetes in middle-aged men. Diabetologia. 2004;47:1403-1410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 190. | Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286-1292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 816] [Cited by in F6Publishing: 865] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 191. | Mendall MA, Patel P, Ballam L, Strachan D, Northfield TC. C reactive protein and its relation to cardiovascular risk factors: a population based cross sectional study. BMJ. 1996;312:1061-1065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 581] [Cited by in F6Publishing: 570] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 192. | Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972-978. [PubMed] [Cited in This Article: ] |
| 193. | Koenig W, Sund M, Fröhlich M, Fischer HG, Löwel H, Döring A, Hutchinson WL, Pepys MB. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1275] [Cited by in F6Publishing: 1244] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 194. | Del Cañizo-Gómez FJ, Moreira-Andrés MN. C-reactive protein in obese patients with type 2 diabetes mellitus and metabolic syndrome. 2008;117. [Cited in This Article: ] |
| 195. | Del Cañizo-Gómez FJ, Fernández-Pérez C, Pajuelo-Fernández FJ. Waist circumference is the major determinant of elevated C-reactive protein in type 2 diabetes mellitus subjects with metabolic syndrome. 2008;S989-S990. [Cited in This Article: ] |
| 196. | Baba MM, Kolawole BA, Ikem RT, Arogundade FA, Yusuph H, Gezawa ID. Serum C-reactive protein in Nigerians with type II diabetes mellitus. Nig Q J Hosp Med. 2010;20:108-113. [PubMed] [Cited in This Article: ] |
| 197. | Hirata A, Ohnaka K, Morita M, Toyomura K, Kono S, Yamamoto K, Adachi M, Kawate H, Takayanagi R. Behavioral and clinical correlates of high-sensitivity C-reactive protein in Japanese men and women. Clin Chem Lab Med. 2012;50:1469-1476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 198. | Nesto R. C-reactive protein, its role in inflammation, Type 2 diabetes and cardiovascular disease, and the effects of insulin-sensitizing treatment with thiazolidinediones. Diabet Med. 2004;21:810-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 199. | Vengen IT, Dale AC, Wiseth R, Midthjell K, Videm V. Neopterin predicts the risk for fatal ischemic heart disease in type 2 diabetes mellitus: long-term follow-up of the HUNT 1 study. Atherosclerosis. 2009;207:239-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 200. | Coppola G, Corrado E, Muratori I, Tantillo R, Vitale G, Lo Coco L, Novo S. Increased levels of C-reactive protein and fibrinogen influence the risk of vascular events in patients with NIDDM. Int J Cardiol. 2006;106:16-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 201. | Nyström T, Nygren A, Sjöholm A. Persistent endothelial dysfunction is related to elevated C-reactive protein (CRP) levels in Type II diabetic patients after acute myocardial infarction. Clin Sci (Lond). 2005;108:121-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 202. | Hsieh MC, Tien KJ, Chang SJ, Perng DS, Hsiao JY, Chen YW, Chang YH, Kuo HW, Lin PC. High-sensitivity C-reactive protein and silent myocardial ischemia in Chinese with type 2 diabetes mellitus. Metabolism. 2008;57:1533-1538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 203. | Soinio M, Marniemi J, Laakso M, Lehto S, Rönnemaa T. High-sensitivity C-reactive protein and coronary heart disease mortality in patients with type 2 diabetes: a 7-year follow-up study. Diabetes Care. 2006;29:329-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 204. | Asegaonkar SB, Marathe A, Tekade ML, Cherekar L, Bavikar J, Bardapurkar J, Ajay R. High-sensitivity C-reactive protein: a novel cardiovascular risk predictor in type 2 diabetics with normal lipid profile. J Diabetes Complications. 2011;25:368-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 205. | Cox AJ, Agarwal S, M Herrington D, Carr JJ, Freedman BI, Bowden DW. C-reactive protein concentration predicts mortality in type 2 diabetes: the Diabetes Heart Study. Diabet Med. 2012;29:767-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 206. | Biasucci LM, Liuzzo G, Della Bona R, Leo M, Biasillo G, Angiolillo DJ, Abbate A, Rizzello V, Niccoli G, Giubilato S. Different apparent prognostic value of hsCRP in type 2 diabetic and nondiabetic patients with acute coronary syndromes. Clin Chem. 2009;55:365-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 207. | Khatana SA, Taveira TH, Choudhary G, Eaton CB, Wu WC. Change in hemoglobin A(1c) and C-reactive protein levels in patients with diabetes mellitus. J Cardiometab Syndr. 2009;4:76-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 208. | Godsland IF, Elkeles RS, Feher MD, Nugara F, Rubens MB, Richmond W, Khan M, Donovan J, Anyaoku V, Flather MD. Coronary calcification, homocysteine, C-reactive protein and the metabolic syndrome in Type 2 diabetes: the Prospective Evaluation of Diabetic Ischaemic Heart Disease by Coronary Tomography (PREDICT) Study. Diabet Med. 2006;23:1192-1200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 209. | Friedman AN, Hunsicker LG, Selhub J, Bostom AG. Clinical and nutritional correlates of C-reactive protein in type 2 diabetic nephropathy. Atherosclerosis. 2004;172:121-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 210. | Ray I, Datta AK, Mukhopadhyay P, Roy S, Khandakar MR, Maity A. A study of the association of micro-albuminuria and C-reactive protein (CRP) in normotensive diabetic and hypertensive diabetic patients. J Indian Med Assoc. 2011;109:428-429. [PubMed] [Cited in This Article: ] |
| 211. | Del Cañizo-Gómez FJ, Moreira-Andrés MN. Serum high sensitivity C-reactive protein, metabolic syndrome and albuminuria in type 2 diabetes mellitus. 76th Congress of the European Atherosclerosis Society; 2007 June 10-13; Helsinki, Finland; Ireland: Elsevier Ltd., 2007. Atherosclerosis Suppl. 2007;8:144. [Cited in This Article: ] |
| 212. | Friedman AN, Hunsicker LG, Selhub J, Bostom AG. C-reactive protein as a predictor of total arteriosclerotic outcomes in type 2 diabetic nephropathy. Kidney Int. 2005;68:773-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 213. | van der Velde M, Bello AK, Brantsma AH, El Nahas M, Bakker SJ, de Jong PE, Gansevoort RT. Do albuminuria and hs-CRP add to the International Diabetes Federation definition of the metabolic syndrome in predicting outcome? Nephrol Dial Transplant. 2012;27:2275-2283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 214. | Wang LY, Wu SL, Yang XL, Wang TJ, Su LR, Gao JS, Zheng XM, Liu XR. [Association between baseline high sensitivity C-reactive protein level and the first cardio-cerebral vascular event in diabetic population: a prospective cohort study]. Zhonghua Xinxueguanbing Zazhi. 2011;39:749-754. [PubMed] [Cited in This Article: ] |
| 215. | Bruno G, Fornengo P, Novelli G, Panero F, Perotto M, Segre O, Zucco C, Deambrogio P, Bargero G, Perin PC. C-reactive protein and 5-year survival in type 2 diabetes: the Casale Monferrato Study. Diabetes. 2009;58:926-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 216. | Mohan V, Deepa R, Velmurugan K, Premalatha G. Association of C-reactive protein with body fat, diabetes and coronary artery disease in Asian Indians: the Chennai Urban Rural Epidemiology Study (CURES-6). Diabet Med. 2005;22:863-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 217. | Schulze MB, Rimm EB, Li T, Rifai N, Stampfer MJ, Hu FB. C-reactive protein and incident cardiovascular events among men with diabetes. Diabetes Care. 2004;27:889-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 218. | Lowe G, Woodward M, Hillis G, Rumley A, Li Q, Harrap S, Marre M, Hamet P, Patel A, Poulter N. Circulating inflammatory markers and the risk of vascular complications and mortality in people with type 2 diabetes and cardiovascular disease or risk factors: the ADVANCE study. Diabetes. 2014;63:1115-1123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 219. | Viña Rodríguez JJ. Hiperhomocisteinemia, factores de riesgo y afectación cardiovascular en pacientes de más de 65 años ingresados en un servicio de medicina interna [tesis doctoral]. Santa Cruz de Tenerife: Servicio de publicaciones, Universidad de la Laguna, 2004/ 2005; . [Cited in This Article: ] |
| 220. | Lim HS, Heo YR. Plasma total homocysteine, folate, and vitamin B12 status in Korean adults. J Nutr Sci Vitaminol (Tokyo). 2002;48:290-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 221. | Friedman AN, Bostom AG, Selhub J, Levey AS, Rosenberg IH. The kidney and homocysteine metabolism. J Am Soc Nephrol. 2001;12:2181-2189. [PubMed] [Cited in This Article: ] |
| 222. | Balogh E, Czuriga I, Bereczky Z, Boda K, Koszegi Z, Kónya C, Császár A, Muszbek L, Edes I, Ferdinandy P. [Increase of homocysteine in cardiovascular diseases in Hungary]. Orv Hetil. 2006;147:1685-1690. [PubMed] [Cited in This Article: ] |
| 223. | Rudy A, Kowalska I, Straczkowski M, Kinalska I. Homocysteine concentrations and vascular complications in patients with type 2 diabetes. Diabetes Metab. 2005;31:112-117. [PubMed] [Cited in This Article: ] |
| 224. | Vangelder E, Delecourt F, Cardozo MB, Dhondt JL, Forzy G. [Hyperhomocysteinaemia and type 2 diabetes]. Ann Biol Clin (Paris). 2006;64:485-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 225. | Otieno CF, Vaghela V, Ogola EN, Amayo EO. Patterns of homocysteine in Kenyans with type 2 diabetes without overt cardiovascular disease at Kenyatta National Hospital, Nairobi. East Afr Med J. 2005;82:S180-S183. [PubMed] [Cited in This Article: ] |
| 226. | de Luis DA, Fernandez N, Arranz ML, Aller R, Izaola O, Romero E. Total homocysteine levels relation with chronic complications of diabetes, body composition, and other cardiovascular risk factors in a population of patients with diabetes mellitus type 2. J Diabetes Complications. 2005;19:42-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 227. | Song Y, Cook NR, Albert CM, Van Denburgh M, Manson JE. Effect of homocysteine-lowering treatment with folic Acid and B vitamins on risk of type 2 diabetes in women: a randomized, controlled trial. Diabetes. 2009;58:1921-1928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 228. | Stehouwer CD, Gall MA, Hougaard P, Jakobs C, Parving HH. Plasma homocysteine concentration predicts mortality in non-insulin-dependent diabetic patients with and without albuminuria. Kidney Int. 1999;55:308-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 229. | Drzewoski J, Czupryniak L, Chwatko G, Bald E. Hyperhomocysteinemia in poorly controlled type 2 diabetes patients. Diabetes Nutr Metab. 2000;13:319-324. [PubMed] [Cited in This Article: ] |
| 230. | Chait A, Malinow MR, Nevin DN, Morris CD, Eastgard RL, Kris-Etherton P, Pi-Sunyer FX, Oparil S, Resnick LM, Stern JS. Increased dietary micronutrients decrease serum homocysteine concentrations in patients at high risk of cardiovascular disease. Am J Clin Nutr. 1999;70:881-887. [PubMed] [Cited in This Article: ] |
| 231. | Waluś-Miarka M, Czarnecka D, Kawecka-Jaszcz K, Małek A, Sztefko K, Idzior-Waluś B, Miarka P, Sieradzki J. [Arterial wave velocity and coronary risk factors in patients with type-2 diabetes]. Przegl Lek. 2005;62:46-48. [PubMed] [Cited in This Article: ] |
| 232. | Okada E, Oida K, Tada H, Asazuma K, Eguchi K, Tohda G, Kosaka S, Takahashi S, Miyamori I. Hyperhomocysteinemia is a risk factor for coronary arteriosclerosis in Japanese patients with type 2 diabetes. Diabetes Care. 1999;22:484-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 233. | Braun LT. Cardiovascular disease: strategies for risk assessment and modification. J Cardiovasc Nurs. 2006;21:S20-S42; quiz S43-S45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 234. | Tarkun I, Cetinarslan B, Cantürk Z, Tarkun P, Agaçdiken A, Komsuoglu B. Homocysteine concentrations in type 2 diabetic patients with silent myocardial ischemia: a predictive marker. J Diabetes Complications. 2004;18:165-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 235. | Ndrepepa G, Kastrati A, Braun S, Koch W, Kölling K, Mehilli J, Schömig A. A prospective cohort study of predictive value of homocysteine in patients with type 2 diabetes and coronary artery disease. Clin Chim Acta. 2006;373:70-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 236. | Hoogeveen EK, Kostense PJ, Jager A, Heine RJ, Jakobs C, Bouter LM, Donker AJ, Stehouwer CD. Serum homocysteine level and protein intake are related to risk of microalbuminuria: the Hoorn Study. Kidney Int. 1998;54:203-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 104] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 237. | Kuswardhani RA, Suastika K. Age and homocystein were risk factor for peripheral arterial disease in elderly with type 2 diabetes mellitus. Acta Med Indones. 2010;42:94-99. [PubMed] [Cited in This Article: ] |
| 238. | Cohen JA, Jeffers BW, Stabler S, Schrier RW, Estascio R. Increasing homocysteine levels and diabetic autonomic neuropathy. Auton Neurosci. 2001;87:268-273. [PubMed] [Cited in This Article: ] |
| 239. | Yücel I, Yücel G, Müftüoglu F. Plasma homocysteine levels in noninsulin-dependent diabetes mellitus with retinopathy and neovascular glaucoma. Int Ophthalmol. 2004;25:201-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 240. | Aydin E, Demir HD, Ozyurt H, Etikan I. Association of plasma homocysteine and macular edema in type 2 diabetes mellitus. Eur J Ophthalmol. 2008;18:226-232. [PubMed] [Cited in This Article: ] |
| 241. | Ebesunun MO, Obajobi EO. Elevated plasma homocysteine in type 2 diabetes mellitus: a risk factor for cardiovascular diseases. Pan Afr Med J. 2012;12:48. [PubMed] [Cited in This Article: ] |
| 242. | Rafnsson SB, Saravanan P, Bhopal RS, Yajnik CS. Is a low blood level of vitamin B12 a cardiovascular and diabetes risk factor? A systematic review of cohort studies. Eur J Nutr. 2011;50:97-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 243. | Brazionis L, Walker KZ, Itsiopoulos C, O’Dea K. Plasma retinol: a novel marker for cardiovascular disease mortality in Australian adults. Nutr Metab Cardiovasc Dis. 2012;22:914-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 244. | Kassab A, Leban N, Ferchichi S, Feki M, Ben Limem H, Chaeib L, Miled A. [Oxidant stress and cardiovascular absolute risk in Tunisian type 2 diabetes]. Ann Biol Clin (Paris). 2008;66:151-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 245. | Ferrarezi DA, Bellili-Muñoz N, Dubois-Laforgue D, Cheurfa N, Lamri A, Reis AF, Le Feuvre C, Roussel R, Fumeron F, Timsit J. Allelic variations of the vitamin D receptor (VDR) gene are associated with increased risk of coronary artery disease in type 2 diabetics: the DIABHYCAR prospective study. Diabetes Metab. 2013;39:263-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 246. | Brewer LC, Michos ED, Reis JP. Vitamin D in atherosclerosis, vascular disease, and endothelial function. Curr Drug Targets. 2011;12:54-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 247. | Molinari C, Uberti F, Grossini E, Vacca G, Carda S, Invernizzi M, Cisari C. 1α,25-dihydroxycholecalciferol induces nitric oxide production in cultured endothelial cells. Cell Physiol Biochem. 2011;27:661-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 248. | Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, Proctor BM, Petty M, Chen Z, Schechtman KB. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 274] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 249. | Tare M, Emmett SJ, Coleman HA, Skordilis C, Eyles DW, Morley R, Parkington HC. Vitamin D insufficiency is associated with impaired vascular endothelial and smooth muscle function and hypertension in young rats. J Physiol. 2011;589:4777-4786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 250. | Pilz S, Tomaschitz A, März W, Drechsler C, Ritz E, Zittermann A, Cavalier E, Pieber TR, Lappe JM, Grant WB. Vitamin D, cardiovascular disease and mortality. Clin Endocrinol (Oxf). 2011;75:575-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 251. | Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65:1005-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 351] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 252. | Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1581] [Cited by in F6Publishing: 1598] [Article Influence: 99.9] [Reference Citation Analysis (0)] |
| 253. | Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95:91-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 268] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 254. | Duranton F, Rodriguez-Ortiz ME, Duny Y, Rodriguez M, Daurès JP, Argilés A. Vitamin D treatment and mortality in chronic kidney disease: a systematic review and meta-analysis. Am J Nephrol. 2013;37:239-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 255. | Bittner V, Wenger NK, Waters DD, DeMicco DA, Messig M, LaRosa JC. Vitamin D levels do not predict cardiovascular events in statin-treated patients with stable coronary disease. Am Heart J. 2012;164:387-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 256. | Schöttker B, Ball D, Gellert C, Brenner H. Serum 25-hydroxyvitamin D levels and overall mortality. A systematic review and meta-analysis of prospective cohort studies. Ageing Res Rev. 2013;12:708-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 257. | Pilz S, Kienreich K, Tomaschitz A, Lerchbaum E, Meinitzer A, März W, Zittermann A, Dekker JM. Vitamin D and cardiovascular disease: update and outlook. Scand J Clin Lab Invest Suppl. 2012;243:83-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 9] [Reference Citation Analysis (0)] |
| 258. | Joergensen C, Reinhard H, Schmedes A, Hansen PR, Wiinberg N, Petersen CL, Winther K, Parving HH, Jacobsen PK, Rossing P. Vitamin D levels and asymptomatic coronary artery disease in type 2 diabetic patients with elevated urinary albumin excretion rate. Diabetes Care. 2012;35:168-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 259. | Joergensen C, Gall MA, Schmedes A, Tarnow L, Parving HH, Rossing P. Vitamin D levels and mortality in type 2 diabetes. Diabetes Care. 2010;33:2238-2243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 260. | Braun TR, Been LF, Blackett PR, Sanghera DK. Vitamin D Deficiency and Cardio-Metabolic Risk in a North Indian Community with Highly Prevalent Type 2 Diabetes. J Diabetes Metab. 2012;3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 261. | Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 389] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 262. | Alkharfy KM, Al-Daghri NM, Sabico SB, Al-Othman A, Moharram O, Alokail MS, Al-Saleh Y, Kumar S, Chrousos GP. Vitamin D supplementation in patients with diabetes mellitus type 2 on different therapeutic regimens: a one-year prospective study. Cardiovasc Diabetol. 2013;12:113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 263. | Dworzański W, Opielak G, Burdan F. [Side effects of caffeine]. Pol Merkur Lekarski. 2009;27:357-361. [PubMed] [Cited in This Article: ] |
| 264. | Ranheim T, Halvorsen B. Coffee consumption and human health--beneficial or detrimental?--Mechanisms for effects of coffee consumption on different risk factors for cardiovascular disease and type 2 diabetes mellitus. Mol Nutr Food Res. 2005;49:274-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 159] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 265. | de la Vega MJ, Santolaria F, González-Reimers E, Alemán MR, Milena A, Martínez-Riera A, González-García C. High prevalence of hyperhomocysteinemia in chronic alcoholism: the importance of the thermolabile form of the enzyme methylenetetrahydrofolate reductase (MTHFR). Alcohol. 2001;25:59-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 266. | Carmel R, James SJ. Alcohol abuse: an important cause of severe hyperhomocysteinemia. Nutr Rev. 2002;60:215-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 267. | Sakuta H, Suzuki T, Katayama Y, Yasuda H, Ito T. Heavy alcohol intake, homocysteine and Type 2 diabetes. Diabet Med. 2005;22:1359-1363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 268. | Aarsand AK, Carlsen SM. Folate administration reduces circulating homocysteine levels in NIDDM patients on long-term metformin treatment. J Intern Med. 1998;244:169-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 269. | Giuliano FA, Leriche A, Jaudinot EO, de Gendre AS. Prevalence of erectile dysfunction among 7689 patients with diabetes or hypertension, or both. Urology. 2004;64:1196-1201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 187] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 270. | Kalter-Leibovici O, Wainstein J, Ziv A, Harman-Bohem I, Murad H, Raz I. Clinical, socioeconomic, and lifestyle parameters associated with erectile dysfunction among diabetic men. Diabetes Care. 2005;28:1739-1744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 271. | Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2769] [Cited by in F6Publishing: 2515] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 272. | Gazzaruso C, Giordanetti S, De Amici E, Bertone G, Falcone C, Geroldi D, Fratino P, Solerte SB, Garzaniti A. Relationship between erectile dysfunction and silent myocardial ischemia in apparently uncomplicated type 2 diabetic patients. Circulation. 2004;110:22-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 261] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 273. | Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294:2996-3002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 642] [Cited by in F6Publishing: 594] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 274. | Frantzen J, Speel TG, Kiemeney LA, Meuleman EJ. Cardiovascular risk among men seeking help for erectile dysfunction. Ann Epidemiol. 2006;16:85-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 275. | Meena BL, Kochar DK, Agarwal TD, Choudhary R, Kochar A. Association between erectile dysfunction and cardiovascular risk in individuals with type-2 diabetes without overt cardiovascular disease. Int J Diabetes Dev Ctries. 2009;29:150-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 276. | Ma RC, So WY, Yang X, Yu LW, Kong AP, Ko GT, Chow CC, Cockram CS, Chan JC, Tong PC. Erectile dysfunction predicts coronary heart disease in type 2 diabetes. J Am Coll Cardiol. 2008;51:2045-2050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 277. | Yamada T, Hara K, Umematsu H, Suzuki R, Kadowaki T. Erectile dysfunction and cardiovascular events in diabetic men: a meta-analysis of observational studies. PLoS One. 2012;7:e43673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 278. | Sorenson M, Grant WB. Does vitamin D deficiency contribute to erectile dysfunction? Dermatoendocrinol. 2012;4:128-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 279. | Diabetes mellitus: a major risk factor for cardiovascular disease. A joint editorial statement by the American Diabetes Association; The National Heart, Lung, and Blood Institute; The Juvenile Diabetes Foundation International; The National Institute of Diabetes and Digestive and Kidney Diseases; and The American Heart Association. Circulation. 1999;100:1132-1133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 186] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 280. | Levy AP. Haptoglobin: a major susceptibility gene for diabetic cardiovascular disease. Isr Med Assoc J. 2004;6:308-310. [PubMed] [Cited in This Article: ] |
| 281. | Asleh R, Levy AP. In vivo and in vitro studies establishing haptoglobin as a major susceptibility gene for diabetic vascular disease. Vasc Health Risk Manag. 2005;1:19-28. [PubMed] [Cited in This Article: ] |
| 282. | Spagnuolo MS, Cigliano L, D’Andrea LD, Pedone C, Abrescia P. Assignment of the binding site for haptoglobin on apolipoprotein A-I. J Biol Chem. 2005;280:1193-1198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 283. | Levy AP, Hochberg I, Jablonski K, Resnick HE, Lee ET, Best L, Howard BV. Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes: The Strong Heart Study. J Am Coll Cardiol. 2002;40:1984-1990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 220] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 284. | Levy AP, Larson MG, Corey D, Lotan R, Vita JA, Benjamin EJ. Haptoglobin phenotype and prevalent coronary heart disease in the Framingham offspring cohort. Atherosclerosis. 2004;172:361-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 285. | Suleiman M, Aronson D, Asleh R, Kapeliovich MR, Roguin A, Meisel SR, Shochat M, Sulieman A, Reisner SA, Markiewicz W. Haptoglobin polymorphism predicts 30-day mortality and heart failure in patients with diabetes and acute myocardial infarction. Diabetes. 2005;54:2802-2806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 286. | Adams JN, Cox AJ, Freedman BI, Langefeld CD, Carr JJ, Bowden DW. Genetic analysis of haptoglobin polymorphisms with cardiovascular disease and type 2 diabetes in the Diabetes Heart Study. Cardiovasc Diabetol. 2013;12:31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 287. | Davignon J, Gregg RE, Sing CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988;8:1-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1274] [Cited by in F6Publishing: 1240] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 288. | Siest G, Pillot T, Régis-Bailly A, Leininger-Muller B, Steinmetz J, Galteau MM, Visvikis S. Apolipoprotein E: an important gene and protein to follow in laboratory medicine. Clin Chem. 1995;41:1068-1086. [PubMed] [Cited in This Article: ] |
| 289. | Knouff C, Hinsdale ME, Mezdour H, Altenburg MK, Watanabe M, Quarfordt SH, Sullivan PM, Maeda N. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J Clin Invest. 1999;103:1579-1586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 234] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 290. | Laakso M, Kesäniemi A, Kervinen K, Jauhiainen M, Pyörälä K. Relation of coronary heart disease and apolipoprotein E phenotype in patients with non-insulin dependent diabetes. BMJ. 1991;303:1159-1162. [PubMed] [Cited in This Article: ] |
| 291. | Ukkola O, Kervinen K, Salmela PI, von Dickhoff K, Laakso M, Kesäniemi YA. Apolipoprotein E phenotype is related to macro- and microangiopathy in patients with non-insulin-dependent diabetes mellitus. Atherosclerosis. 1993;101:9-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 292. | Guangda X, Bangshun X, Xiujian L, Yangzhong H. Apovarepsilon(4) allele increases the risk for exercise-induced silent myocardial ischemia in non-insulin-dependent diabetes mellitus. Atherosclerosis. 1999;147:293-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 293. | Guangda X, Yuhua W. Apolipoprotein e4 allele and endothelium-dependent arterial dilation in Type 2 diabetes mellitus without angiopathy. Diabetologia. 2003;46:514-519. [PubMed] [Cited in This Article: ] |
| 294. | Chaudhary R, Likidlilid A, Peerapatdit T, Tresukosol D, Srisuma S, Ratanamaneechat S, Sriratanasathavorn C. Apolipoprotein E gene polymorphism: effects on plasma lipids and risk of type 2 diabetes and coronary artery disease. Cardiovasc Diabetol. 2012;11:36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 295. | Bruns GA, Karathanasis SK, Breslow JL. Human apolipoprotein A-I--C-III gene complex is located on chromosome 11. Arteriosclerosis. 1984;4:97-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 92] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 296. | Li WW, Dammerman MM, Smith JD, Metzger S, Breslow JL, Leff T. Common genetic variation in the promoter of the human apo CIII gene abolishes regulation by insulin and may contribute to hypertriglyceridemia. J Clin Invest. 1995;96:2601-2605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 203] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 297. | Chen M, Breslow JL, Li W, Leff T. Transcriptional regulation of the apoC-III gene by insulin in diabetic mice: correlation with changes in plasma triglyceride levels. J Lipid Res. 1994;35:1918-1924. [PubMed] [Cited in This Article: ] |
| 298. | Kliewer SA, Xu HE, Lambert MH, Willson TM. Peroxisome proliferator-activated receptors: from genes to physiology. Recent Prog Horm Res. 2001;56:239-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 299. | Hertz R, Bishara-Shieban J, Bar-Tana J. Mode of action of peroxisome proliferators as hypolipidemic drugs. Suppression of apolipoprotein C-III. J Biol Chem. 1995;270:13470-13475. [PubMed] [Cited in This Article: ] |
| 300. | Staels B, Vu-Dac N, Kosykh VA, Saladin R, Fruchart JC, Dallongeville J, Auwerx J. Fibrates downregulate apolipoprotein C-III expression independent of induction of peroxisomal acyl coenzyme A oxidase. A potential mechanism for the hypolipidemic action of fibrates. J Clin Invest. 1995;95:705-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 329] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 301. | Waterworth DM, Talmud PJ, Bujac SR, Fisher RM, Miller GJ, Humphries SE. Contribution of apolipoprotein C-III gene variants to determination of triglyceride levels and interaction with smoking in middle-aged men. Arterioscler Thromb Vasc Biol. 2000;20:2663-2669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 302. | Olivieri O, Martinelli N, Sandri M, Bassi A, Guarini P, Trabetti E, Pizzolo F, Girelli D, Friso S, Pignatti PF. Apolipoprotein C-III, n-3 polyunsaturated fatty acids, and “insulin-resistant” T-455C APOC3 gene polymorphism in heart disease patients: example of gene-diet interaction. Clin Chem. 2005;51:360-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 303. | Ling C, Groop L. Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes. 2009;58:2718-2725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 422] [Cited by in F6Publishing: 437] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 304. | Fu LH, Cong B, Zhen YF, Li SJ, Ma CL, Ni ZY, Zhang GZ, Zuo M, Yao YX. [Methylation status of the IL-10 gene promoter in the peripheral blood mononuclear cells of rheumatoid arthritis patients]. Yi Chuan. 2007;29:1357-1361. [PubMed] [Cited in This Article: ] |
| 305. | Miao F, Gonzalo IG, Lanting L, Natarajan R. In vivo chromatin remodeling events leading to inflammatory gene transcription under diabetic conditions. J Biol Chem. 2004;279:18091-18097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 270] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 306. | Hofmann MA, Schiekofer S, Kanitz M, Klevesath MS, Joswig M, Lee V, Morcos M, Tritschler H, Ziegler R, Wahl P. Insufficient glycemic control increases nuclear factor-kappa B binding activity in peripheral blood mononuclear cells isolated from patients with type 1 diabetes. Diabetes Care. 1998;21:1310-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 121] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 307. | Shanmugam N, Reddy MA, Guha M, Natarajan R. High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes. 2003;52:1256-1264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 392] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 308. | El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205:2409-2417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 752] [Cited by in F6Publishing: 759] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 309. | Brasacchio D, Okabe J, Tikellis C, Balcerczyk A, George P, Baker EK, Calkin AC, Brownlee M, Cooper ME, El-Osta A. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes. 2009;58:1229-1236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 389] [Cited by in F6Publishing: 394] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 310. | Keating ST, El-Osta A. Epigenetic changes in diabetes. Clin Genet. 2013;84:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 6.0] [Reference Citation Analysis (1)] |