Preoperative mediastinal lymph node staging for non-small cell lung cancer: 2014 update of the 2007 ESTS guidelines
Introduction
For patients with non-small cell lung cancer (NSCLC) and no systemic metastasis, mediastinal staging is very important as it provides accurate information on the extent of the disease, it guides the choice of treatment and determines the patient’s prognosis.
In 2007, the European Society of Thoracic Surgeons (ESTS) published an algorithm on preoperative mediastinal staging based on the current available literature (1). These guidelines integrated imaging, endoscopic and surgical techniques. They were widely used and have been prospectively validated. Their negative predictive value is 0.94 (2).
New insights on the importance of restaging and techniques for mediastinal restaging have become available. Therefore, the ESTS Council approved the initiative by the working group to revise and update the previous guidelines on mediastinal staging.
Methodology
There were several meetings of the working group. The project was discussed in the Council at the ESTS meeting in Essen (June 2012). There were several meetings (Essen, Zürich, Brussels and Birmingham) where the participants presented their experience and discussed the relevant literature published since 2007. Initial findings were presented and discussed at the ESTS meeting in Birmingham (May 2013). The final paper was put on the website for discussion by all ESTS members. Their remarks were discussed and included in the final manuscript.
For recommendations, a level of evidence and grading of recommendation is given. This was adapted from the Infectious Disease Society of American-United States Public Health Service Grading System (Table 1) (3).

Full table
It is evident that both in primary staging and restaging, not every technique is available in every centre. Therefore, staging and restaging techniques can differ between different countries and centres.
Rationale for preoperative mediastinal nodal staging
The current guidelines for treatment of lung cancer are determined by the clinical status of the mediastinal nodes. The aim of mediastinal staging is to exclude with the highest certainty and the lowest morbidity patients with mediastinal nodal disease since these patients will not benefit from upfront surgery (4,5).
There is controversy regarding the best treatment of N2 disease because of the heterogeneity of nodal involvement. Also patient and tumour characteristics and extent of resection plays a role in the selection of treatment modality for these patients.
There is a subgroup of patients with pretreatment histologically proven N2 disease who are candidate for surgical multimodality treatment. These patients are treated with induction chemotherapy or induction chemoradiotherapy. In case of downstaging of the mediastinal lymph nodes or major response in those lymph nodes and in the tumour, resection with systematic nodal dissection can be performed with acceptable morbidity and mortality and rewarding 5-year survival. There are several prognostic indicators, some of them are related to the primary tumour and others are related to the extent of nodal disease. To include patients for surgical multimodality treatment, the disease should be initially technically resectable. Excluded for surgical multimodality are patients with unresectable disease such as extracapsular disease (can be clearly visualized by mediastinoscopy), or bulky N2 disease based on CT. Fit patients with extracapsular disease and/or bulky N2 disease should be treated with definitive chemoradiotherapy.
Bulky N2 disease is not well defined but it correlates with the radiographic group A, as described in the American College of Chest Physicians (ACCP) Evidence-based Clinical Practice Guidelines (6). This group is defined as mediastinal infiltration where the discrete lymph nodes cannot be distinguished or measured. Bulky is not strictly related to the size of the lymph nodes, but it is considered by this committee that lymph nodes larger than 25 mm short axis will also be defined as bulky disease (level V). Bulky disease can be restricted to a single station but usually represents multistation or multiple zonal involvement. Since this paper deals with preoperative lymph node staging, techniques to obtain histology in bulky mediastinal nodal disease are beyond the scope of this article.
Preoperative mediastinal lymph node staging
Several techniques are available and their use depends on local availability and local expertise.
These techniques include:
- Imaging techniques;
- Endoscopic techniques;
- Surgical techniques.
Although we should aim for the test with the highest sensitivity and NPV, the working group considers a rate of unforeseen pN2 disease of 10% as acceptable. After thorough mediastinal staging this unforeseen pN2 is mostly single station resectable nodal disease.
Imaging techniques
Chest CT-scan
Computed tomography remains important in lung cancer imaging. However, due to its low sensitivity (55%) and specificity (81%) it is impossible to solely rely on CT-scan (6). CT-scan may help us in selecting the appropriate procedure for tissue sampling due to the anatomical images it provides.
PET-CT scan
The addition of PET to CT results in more accurate lymph node staging than CT alone with an overall sensitivity of 80-90% and specificity of 85-95%. PET-CT has a high NPV for detecting mediastinal nodal disease in peripherally located NSCLC. Exceptions include:
- Suspected N1 nodes;
- Tumour >3 cm;
- Centrally located tumour without suspected nodes on CT or PET scan.
In a study from Japan (7), 30% of 143 patients with N1 disease on CT-scan (lymph node short axis >1 cm) were found to have pathologic N2-N3.
A recent meta-analysis (8) has shown that the negative predictive value of PET-CT for tumours ≤3 cm was 94% (649 patients) compared to 89% for tumours >3 cm (130 patients) staged as T2 (6th edition of TNM). This finding was confirmed in a recent prospective study from Spain (9). For peripheral tumours ≤3 cm the negative predictive value of PET-CT was 92% while it was 85% for tumours >3 cm. Based on these studies, we now recommend that for peripheral tumours (outer third of the lung) ≤3 cm without enlarged (hilar and/or mediastinal) lymph nodes on CT and with PET-negative nodes, further mediastinal staging can be omitted. There was a substantial difference in rate of mediastinal nodal disease between adenocarcinoma and other tumour histology (risk ratio 2.72). Also high FDG uptake in the primary lesion was associated with greater risk of occult nodal metastasis. For tumours >3 cm (mainly adenocarcinoma with high FDG uptake) further mediastinal staging techniques providing histology should be considered.
Lee et al. (10) examined the prevalence of pathologic N2 disease in patients with clinical stage I NSCLC (6th edition of TNM version) with negative mediastinum on PET and CT. In 2.9% of peripheral tumours (outer third of lung) N2 disease was found, while the prevalence of N2 disease was 21.6% in central tumours.
Diffusion-weighted magnetic resonance imaging
Advances in MRI technology have allowed acquisition of diffusion-weighted MRI (DWI), which provides excellent tissue contrast because of the difference in the diffusion of water molecules among tissues. The technique yields qualitative and quantitative information that reflects changes at a cellular level and provides unique insights about tumour cellularity and the integrity of cell membranes. In a recent meta-analysis (11) the accuracy of DWI and 18F-FDG PET/CT was evaluated. The pooled sensitivity for DWI was 0.95 (95% CI, 0.85-0.98) and significantly better than for FDG-PET/CT 0.89 (89% CI, 0.85-0.91). However, at this moment there are no large prospective studies comparing the value of DWI and FDG-PET and it is too early to determine the true value of DWI in nodal staging in patients with NSCLC.
Endoscopic techniques
Conventional TBNA
Although the conventional TBNA technique has been available for almost three decades, its use in routine clinical practice has only been adopted by a minority (10-15%) of pulmonologists for mediastinal nodal staging of patients with potentially resectable stage I-III lung cancer. Major reasons for its underuse are its dependency on nodal size (>15-20 mm short axis on CT scan) and operator skills. Meta-analyses reported a sensitivity of 78% and a false negative rate of 28% for conventional TBNA in clinical N2 disease with high disease prevalence of 81% (12,13). A conventional blind TBNA is useful if it leads to proof of N3 disease, but too often does not exclude N3 disease in cases of proven N2 disease.
Endoscopic ultrasonography: EUS-FNA and EBUS-TBNA
Practical aspects
Although E(B)US-TBNA is performed in some centers under general anesthesia, EBUS and EUS are more often performed in an outpatient setting under local anesthesia with moderate sedation.
EBUS is able to visualise superior and inferior mediastinal LNs at stations 2R/2L, 4R/4L and 7, as well as hilar LNs at stations 10, 11, and even 12, as described on the new LN map (14). EUS particularly visualises superior mediastinal lymph nodes in station 4L, and inferior mediastinal nodes in stations 7, 8 and 9, as described on the new LN map (Rusch 2009). Thus, EUS-FNA complements other techniques, as several of these LNs (stations 8 and 9) are not accessible by EBUS-TBNA or mediastinoscopy. Although some expert centres considered EUS-FNA of lymph nodes in stations 5 or 6, currently available data are limited and therefore we do not recommend routine use of this procedure for this indication (15).
It is possible to visualize and sample lymph nodes with a short axis of >5 mm and the optimal number of aspirations per station has been reported to be three (16). When mediastinal nodal staging is required, systematic nodal sampling is feasible by endosonography. Indeed, several endosonography series have shown a mean or median number of sampled mediastinal nodal stations of 3 to 4 per patient (17-22). Nodal stations 4R, 4L, and 7 should always be sought during the endosonographic examination and described in the medical report. In addition the largest node measuring >5 mm on ultrasonography within each of these stations as well as FDG avid nodes within each of these nodal stations should be sampled for pathological analysis. On indication nodal station 10R and 10L can be biopsied. To avoid contamination while using one single needle for an EBUS or EUS procedure, the order of nodal sampling should begin at the level of N3 nodes followed by N2 nodes before ending with N1 nodes.
Performance characteristics
Several meta-analyses on EUS-FNA alone, EBUS-TBNA alone, and combined EUS+EBUS reported a pooled sensitivity of 83% to 94% for mediastinal staging of lung cancer (Table 2) (23-27). Only one randomized controlled trial (Aster trial, 17) has been performed, comparing the two staging strategies proposed in the ESTS 2007 guidelines (either mediastinoscopy, or alternatively endosonography followed by mediastinoscopy) (1). There was no difference in sensitivity or NPV when mediastinoscpy was compared with endoscopic staging. However, the staging strategy starting with combined endosonography and if negative combining it with surgical staging has proven to detect significantly more mediastinal nodal N2/3 disease compared to mediastinoscopy alone (17). Another consequence is that the implementation of endosonography for baseline mediastinal nodal staging clearly reduces the need for mediastinoscopy (28). On the other hand, the negative likelihood ratio reported by three of the meta-analyses is 0.13 to 0.15 (Table 2) (25-27). This implies that the probability of having mediastinal nodal involvement for any individual patient with a negative endosonography result is 13-15%. This probability based on endosonography alone is in our opinion not low enough to directly proceed to a surgical resection. Therefore in the routine practice we still recommend a preoperative surgical staging procedure (i.e., VAM) in case of a negative endosonography. However, there is evidence coming from prospective studies performed in experienced endosonography centres, that mediastinoscopy may not improve sensitivity after a well-performed negative endosonography with needle aspiration of at least three mediastinal nodal stations in patients with low (<35%) prevalence of mediastinal disease (18,29,30). EBUS-TBNA and EUS-FNA are safe procedures with reported minor complications in <1% of cases (23,24,31). With the rapidly increasing number of procedures, occasional reports of moderate to severe complications have been published, such as pneumothorax requiring chest tube drainage, infection of bronchogenic cyst, empyema, lung and/or mediastinal abscess, and haemopneumomediastinum are published. So far, only one death has been reported related to an EBUS-TBNA procedure (32).

Full table
Surgical staging techniques
Cervical mediastinoscopy
Cervical mediastinoscopy through a pretracheal suprasternal incision was introduced by Carlens in 1959 and further popularized by Pearson in North America. It allows a full mapping of the ipsilateral and contralateral superior mediastinal lymph nodes. Cervical mediastinoscopy is performed under general anaesthesia and can be safely done as an outpatient procedure. For many years it was the gold standard for invasive staging of patients with potentially operable lung cancer. Since 1995, use of video techniques has been introduced leading to video-assisted mediastinoscopy (VAM). VAM clearly improved visualization and teaching (33) since both the trainer and the trainee can share the magnified image on the monitor. For more details on the technique of cervical mediastinoscopy, we refer to a recent publication on this topic (34).
There are only retrospective studies comparing the safety and accuracy of conventional mediastinoscopy with VAM. Although some authors (35-37) found an increase in the number of LN or LN stations biopsied, no difference in sensitivity or NPV was found. In some of these studies a reduction in the complication rate (mainly of recurrent nerve palsy) was observed. Very recently (38), a best evidence topic has been published on the safety and accuracy of VAM compared to conventional mediastinoscopy (Table 3). The authors analysed 108 papers published between 1989 and 2011. There were 5,156 conventional mediastinoscopies and 956 VAMs. Both procedures are safe with no mortality in that time frame and a low morbidity. Although by VAM more lymph node stations are sampled, the negative predictive value and accuracy were identical.
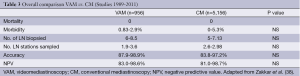
Full table
Although the video-mediastinoscope is not strictly necessary to achieve a thorough, clinically acceptable mediastinoscopy, it has many advantages over the conventional one: larger and clearer images, the possibility to simultaneously share the procedure with trainees and all the personnel in the operative theatre, the possibility to record the operation for future educational uses and discussion, and the possibility to improve its teaching without compromising the safety or accuracy of the procedure. Moreover it allows bimanual dissection with possibilities to perform nodal dissection and removal rather than sampling or biopsy. This is especially important and technically feasible for the subcarinal LN station. After removal of station 7 LNs, the oesophagus can be clearly visualized. The ESTS working group recommends performing VAM.
Video-assisted thoracoscopic surgery (VATS)
Although VATS can reach almost every mediastinal lymph node station, it is more invasive than cervical mediastinoscopy (it needs double lumen intubatio), it is limited by pleural adhesions, and it can only evaluate ipsilateral nodal disease. For the para-aortic lymph nodes (station 6) and the subaortic lymph nodes (station 5), left VATS is a surgical technique that allows obtaining large tissue samples. It is indicated when enlarged PET positive lymph nodes are visualized at level 5 or 6. These lymph node stations cannot be biopsied by routine mediastinoscopy, E(B)US-FNA. An alternative to VATS is the left anterior mediastinotomy. In some experienced centres, extended mediastinoscopy from the mediastinoscopy incision is performed for these lymph node stations and it gives good negative predictive values: 0.89-0.97 (34).
Video-assisted mediastinoscopic lymphadenectomy (VAMLA) transcervical extended mediastinal lymphadenectomy (TEMLA)
During the last decade, two new invasive staging techniques representing more radical methods of mediastinal exploration have been introduced: VAMLA (39) and TEMLA (40). These two techniques aim for a complete removal of all the mediastinal nodes with the surrounding adipose tissue to improve the accuracy of staging. VAMLA is completely performed with the use of the videomediastinoscope whilst TEMLA uses a 5-8 cm collar incision in the neck and elevates the sternum with a hook. The dissection is performed in an open way and with the use of the videomediastinoscope. By VAMLA, the lymph nodes which are usually accessible through mediastinoscopy, are removed. By TEMLA, more lymph node stations are accessible such as the prevascular, the para-aortic, the subaortic and the para-oesophageal lymph node stations. The negative predictive value is very high and approaches 98.7% for TEMLA. Although there is no doubt that the accuracy of mediastinal staging increases when lymphadenectomy is performed compared to nodal biopsy, these techniques have a higher morbidity and mortality. The complications after VAMLA and TEMLA are well recorded and are probably more studied in detail than after CM or VAM. These procedures are performed in very experienced centres. For VAMLA mainly problems with recurrent nerve palsy and important scarring with an impact on subsequent resection are reported (39,41-44). The published data for TEMLA are mainly from one very experienced centre and there are concerns on morbidity and mortality.
For TEMLA and VAMLA we conclude that currently available data regarding its use are limited and, therefore, we do not recommend its use except of clinical trials. We encourage other centres to publish their data with these new staging techniques.
The algorithm for preoperative mediastinal staging is shown in Figure 1. For NSCLC, both for mediastinal as for distant staging, PET or PET-CT is indicated.
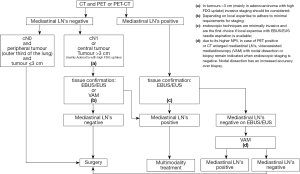
- Direct surgery can be performed if all of these three criteria apply: no suspect lymph node detected by CT or PET, a tumor ≤3 cm (stage IA), located in the outer third of the lung (level IIA).
- In case of enlarged mediastinal lymph nodes on CT or PET-positive lymph nodes, tissue confirmation is indicated. In this case, endosonography (EBUS/EUS) with fine needle aspiration is the first choice (when available) since it is minimally invasive and has a high sensitivity to rule in mediastinal nodal disease (level IA). If negative, video-assisted mediastinoscopy is indicated (level IB). The combined use of endoscopic staging and surgical staging results in the highest accuracy.
For patients with a left upper lobe tumour, surgical staging of the aorto-pulmonary window nodes (if enlarged on CT and/or PET-CT-positive) can be performed (by anterior mediastinotomy, VATS or extended cervical mediastinoscopy) if involvement changes treatment strategy (level V).
- Invasive staging by E(B)US/mediastinoscopy is indicated if at least one of these criteria apply: central lesion, suspect N1 nodes (level IIB). In case of tumors >3 cm (mainly in adenocarcinoma with high FDG uptake) the negative predictive value for mediastinal nodal disease is <90% and invasive staging may be considered (level IIB). Although a high FDG update in the primary tumor is a predictor of N2 disease, the ideal cutoff of SUV value has not yet been determined above which invasive mediastinal nodal staging is required. In addition, the SUV measurement is not yet standardized from one center to another and therefore a visual interpretation of the FDG uptake on PET is to be preferred (Dooms 2010). In all of the above-mentioned cases there is the choice between VAM with biopsy or lymph node dissection or endoscopic staging by EBUS/EUS with fine needle aspiration. The choice depends on local expertise to adhere to minimal requirements for staging (level V). If video-assisted mediastinoscopy is negative, these patients can undergo surgical treatment. They also can undergo surgical treatment after negative EBUS/EUS if the number of nodes explored and the number of needle passes in each node meet the established requirements. Otherwise, surgical exploration is recommended after negative EBUS/EUS.
- If only CT is available, we refer to the algorithm of the 2007 edition of the ESTS guidelines (De Leyn 2007).
We conclude that optimal mediastinal lymph node staging is a truly multidisciplinary process, with a variety of possible techniques, to be performed by experienced hands.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- De Leyn P, Lardinois D, Van Schil PE, et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;32:1-8. [PubMed]
- Gunluoglu MZ, Melek H, Medetoglu B, et al. The validity of preoperative lymph node staging guidelines of European Society of Thoracic Surgeons in non-small-cell lung cancer patients. Eur J Cardiothorac Surg 2011;40:287-90. [PubMed]
- Dykewicz CA, Centers for Disease Control and Prevention (U.S.), Infectious Diseases Society of America, et al. Summary of the Guidelines for Preventing Opportunistic Infections among Hematopoietic Stem Cell Transplant Recipients. Clin Infect Dis 2001;33:139-44. [PubMed]
- Pearson FG, DeLarue NC, Ilves R, et al. Significance of positive superior mediastinal nodes identified at mediastinoscopy in patients with resectable cancer of the lung. J Thorac Cardiovasc Surg 1982;83:1-11. [PubMed]
- Funatsu T, Matsubara Y, Hatakenaka R, et al. The role of mediastinoscopic biopsy in preoperative assessment of lung cancer. J Thorac Cardiovasc Surg 1992;104:1688-95. [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Hishida T, Yoshida J, Nishimura M, et al. Problems in the current diagnostic standards of clinical N1 non-small cell lung cancer. Thorax 2008;63:526-31. [PubMed]
- Wang J, Welch K, Wang L, et al. Negative predictive value of positron emission tomography and computed tomography for stage T1-2N0 non-small-cell lung cancer: a meta-analysis. Clin Lung Cancer 2012;13:81-9. [PubMed]
- Gómez-Caro A, Boada M, Cabañas M, et al. False-negative rate after positron emission tomography/computer tomography scan for mediastinal staging in cI stage non-small-cell lung cancer. Eur J Cardiothorac Surg 2012;42:93-100; discussion 100. [PubMed]
- Lee PC, Port JL, Korst RJ, et al. Risk factors for occult mediastinal metastases in clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:177-81. [PubMed]
- Wu LM, Xu JR, Gu HY, et al. Preoperative mediastinal and hilar nodal staging with diffusion-weighted magnetic resonance imaging and fluorodeoxyglucose positron emission tomography/computed tomography in patients with non-small-cell lung cancer: which is better? J Surg Res 2012;178:304-14. [PubMed]
- Holty JE, Kuschner WG, Gould MK. Accuracy of transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: a meta-analysis. Thorax 2005;60:949-55. [PubMed]
- Detterbeck FC, Jantz MA, Wallace M, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:202S-220S.
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project. A proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- von Bartheld MB, Versteegh MI, Braun J, et al. Transesophageal ultrasound-guided fine-needle aspiration for the mediastinal restaging of non-small cell lung cancer. J Thorac Oncol 2011;6:1510-5. [PubMed]
- Lee HS, Lee GK, Lee HS, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest 2008;134:368-74. [PubMed]
- Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010;304:2245-52. [PubMed]
- Szlubowski A, Zieliński M, Soja J, et al. A combined approach of endobronchial and endoscopic ultrasound-guided needle aspiration in the radiologically normal mediastinum in non-small-cell lung cancer staging--a prospective trial. Eur J Cardiothorac Surg 2010;37:1175-9. [PubMed]
- Herth FJ, Krasnik M, Kahn N, et al. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest 2010;138:790-4. [PubMed]
- Hwangbo B, Lee GK, Lee HS, et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest 2010;138:795-802. [PubMed]
- Block MI. Endobronchial ultrasound for lung cancer staging: how many stations should be sampled? Ann Thorac Surg 2010;89:1582-7. [PubMed]
- Ohnishi R, Yasuda I, Kato T, et al. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal nodal staging of lung cancer. Endoscopy 2011;43:1082-9. [PubMed]
- Micames CG, McCrory DC, Pavey DA, et al. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and metaanalysis. Chest 2007;131:539-48. [PubMed]
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389-96. [PubMed]
- Adams K, Shah PL, Edmonds L, et al. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax 2009;64:757-62. [PubMed]
- Chandra S, Nehra M, Agarwal D, et al. Diagnostic accuracy of endobronchial ultrasound-guided transbronchial needle biopsy in mediastinal lymphadenopathy: a systematic review and meta-analysis. Respir Care 2012;57:384-91. [PubMed]
- Zhang R, Ying K, Shi L, et al. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal lymph node staging of lung cancer: a meta-analysis. Eur J Cancer 2013;49:1860-7. [PubMed]
- Tournoy KG, De Ryck F, Vanwalleghem LR, et al. Endoscopic ultrasound reduces surgical mediastinal staging in lung cancer: a randomized trial. Am J Respir Crit Care Med 2008;177:531-5. [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1.
- Herth FJ, Eberhardt R, Krasnik M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest 2008;133:887-91. [PubMed]
- Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J 2009;33:1156-64. [PubMed]
- Navani N, Brown JM, Nankivell M, et al. Suitability of endobronchial ultrasound-guided transbronchial needle aspiration specimens for subtyping and genotyping of non-small cell lung cancer: a multicenter study of 774 patients. Am J Respir Crit Care Med 2012;185:1316-22. [PubMed]
- Martin-Ucar AE, Chetty GK, Vaughan R, et al. A prospective audit evaluating the role of video-assisted cervical mediastinoscopy (VAM) as a training tool. Eur J Cardiothorac Surg 2004;26:393-5. [PubMed]
- Rami-Porta R, Call S. Invasive staging of mediastinal lymph nodes: mediastinoscopy and remediastinoscopy. Thorac Surg Clin 2012;22:177-89. [PubMed]
- Leschber G, Sperling D, Klemm W, et al. Does video-mediastinoscopy improve the results of conventional mediastinoscopy? Eur J Cardiothorac Surg 2008;33:289-93. [PubMed]
- Anraku M, Miyata R, Compeau C, et al. Video-assisted mediastinoscopy compared with conventional mediastinoscopy: are we doing better? Ann Thorac Surg 2010;89:1577-81. [PubMed]
- Cho JH, Kim J, Kim K, et al. A comparative analysis of video-assisted mediastinoscopy and conventional mediastinoscopy. Ann Thorac Surg 2011;92:1007-11. [PubMed]
- Zakkar M, Tan C, Hunt I. Is video mediastinoscopy a safer and more effective procedure than conventional mediastinoscopy? Interact Cardiovasc Thorac Surg 2012;14:81-4. [PubMed]
- Hürtgen M, Friedel G, Toomes H, et al. Radical video-assisted mediastinoscopic lymphadenectomy (VAMLA)--technique and first results. Eur J Cardiothorac Surg 2002;21:348-51. [PubMed]
- Kuzdzał J, Zieliński M, Papla B, et al. Transcervical extended mediastinal lymphadenectomy--the new operative technique and early results in lung cancer staging. Eur J Cardiothorac Surg 2005;27:384-90; discussion 390.. [PubMed]
- Leschber G, Holinka G, Linder A. Video-assisted mediastinoscopic lymphadenectomy (VAMLA)--a method for systematic mediastinal lymphnode dissection. Eur J Cardiothorac Surg 2003;24:192-5. [PubMed]
- Witte B, Wolf M, Huertgen M, et al. Video-assisted mediastinoscopic surgery: clinical feasibility and accuracy of mediastinal lymph node staging. Ann Thorac Surg 2006;82:1821-7. [PubMed]
- Zielinski M, Szlubowski A, Kołodziej M, et al. Comparison of endobronchial ultrasound and/or endoesophageal ultrasound with transcervical extended mediastinal lymphadenectomy for staging and restaging of non-small-cell lung cancer. J Thorac Oncol 2013;8:630-6. [PubMed]
- Yoo DG, Kim YH, Kim DK, et al. Clinical feasibility and surgical benefits of video-assisted mediastinoscopic lymphadenectomy in the treatment of resectable lung cancer. Eur J Cardiothorac Surg 2011;40:1483-6. [PubMed]


