Complications after tracheal resection and reconstruction: prevention and treatment
Introduction
Tracheal resection and reconstruction (TRR) and laryngotracheal resection and reconstruction (LTRR) can be performed safely and successfully in the majority of patients. Complications—particularly those related to the airway anastomosis—are infrequent but can be devastating. Several factors, including length of resected trachea, preexisting tracheal appliance, prior tracheal resection, and medical comorbidities such as diabetes have been shown to increase the risk of anastomotic complications. Careful preoperative planning, intraoperative technique, and postoperative care mitigate but do not eliminate this risk. Early recognition is the key to effective management of complications. Special emphasis is placed on securing a stable airway, which may in turn require a temporary endotracheal tube or airway appliance. When an anastomotic complication is addressed promptly most patients will go on to have a good outcome. However, the presence of an anastomotic complication increases the risk of perioperative mortality and long-term morbidity (i.e., need for a permanent airway appliance) substantially compared to an uncomplicated operation.
Incidence of complications
Complications following TRR and LTRR can be broadly classified as anastomotic or non-anastomotic. Anastomotic complications include formation of granulation tissue, restenosis of the trachea, varying degrees of anastomotic separation, and fistulae to surrounding structures like the innominate artery [tracheoinnominate fistula (TIF)] and esophagus [tracheoesophageal fistula (TEF)]. Non-anastomotic complication specific to upper airway reconstruction include laryngeal edema and glottic dysfunction, either with regard to phonation or swallowing.
The largest series of TRR and LTRR was published by Wright et al. (1). It includes 901 patients who underwent TRR and LTRR at the Massachusetts General Hospital between 1975 and 2003. The results are summarized in Table 1. Indications for surgery were postintubation tracheal stenosis (PITS) in 589 patients, tumor in 208 patients, idiopathic laryngotracheal stenosis (ILTS) in 83 patients, and TEF in 21 patients. Complications occurred in 164 patients (18.2%). Of these, 81 patients (9.0% of total) had anastomotic complications. LTRR was associated with more complications than TRR. The majority of patients (95%) had a good result from surgery and perioperative mortality was only 1.2% with most deaths occurring during the early years of the study period. Similar results have been reported in recent smaller series by Mutrie et al. (2), Bibas et al. (3), and Piazza et al. (4). Overall, rates of complications both anastomotic and otherwise have decreased from earlier series (5-7), reflecting improvement in patient selection and operative technique.
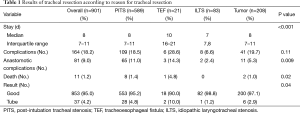
Full table
Of the 81 patients in Wright’s series who had anastomotic complications, seven (1%) had granulation tissue form at the anastomosis, 37 (4%) had restenosis, and 37 (4%) had separation. The incidence of granulation tissue formation has decreased markedly from earlier series from the same institution owing to a change in surgical technique (8). Fistula formation is fortunately a rare event. Three patients developed TIF and three developed TEF. The consequences of TIF, however, are dire.
Non-anastomotic complications occur with moderate frequency following all types of tracheal resection. Wound infection requiring antibiotics with or without drainage occurs in 3–10% of patients (2). Laryngeal dysfunction either in the form of edema or vocal cord palsy increases in frequency the closer the anastomosis is to the vocal cords. Laryngeal dysfunction may also manifest as impaired postoperative swallowing, which is reported in 2–4% of patients (3). Edema requiring intervention is uncommon though precise quantification is difficult. Piazza et al. reported 4 out of 137 patients (3%) who required aggressive management for suspected edema. True injury to the recurrent laryngeal nerve is infrequent (14 of 901 patients in Wright’s series). Laryngeal resection increases the risk considerably. Postoperative hoarseness, however, occurs in 5% of patients (4). For most patients it is impossible to know if hoarseness is a manifestation of laryngeal edema, transient vocal cord palsy, or true nerve injury. Other serious complications such as pneumonia, myocardial infarction, and pulmonary embolism occur at similar or lower rates than those seen following other major thoracic procedures.
Prevention of complications
Several factors have been shown to influence the incidence of anastomotic complications following TRR and LTRR. These include indication for surgery, history of prior resection, presence of an airway appliance, medical comorbidities, and length of trachea resected. Only some of these factors can be modified; however, identifying high-risk individuals improves procedural planning and helps manage patient expectations.
Preoperatively
Institutional and individual surgeon experience has a measurable impact on outcomes following complex airway surgery. Grillo et al. observed that in his personal series of 280 patients the incidence of complications decreased significantly during the second half of the study period (6). The same is certainly true for the anesthesiologists who manage these complex patients in the operating room and the nursing teams who take care of them postoperatively. Tracheal surgery should therefore ideally be performed only in centers with a reasonable operative volume.
Certain patient populations should be approached with caution. In particular, patients with tracheomalacia coexisting with focal stenosis often have suboptimal results from surgery. Similarly, patients with stenosis related to Wegener’s granulomatosis are poor candidates for resection given the unpredictable and relapsing course of the disease. In both groups a permanent airway appliance should be strongly considered if necessary to maintain airway patency. Pediatric patients deserve special consideration because empiric evidence demonstrates that the juvenile trachea tolerates tension less well than that of the adult. Up to 50% of the adult trachea can be resected whereas in children that number is closer to 30% (9).
Patients undergoing resection for TEF or PITS are at significantly higher risk for complications compared to those undergoing resection for tumor or ILTS. This can be explained by a number of factors. Patients with TEF have complex pathology, chronic illness, and a substantial burden of comorbid conditions. Like patients with PITS, they also tend to have a long segment of involved trachea. Inflammation around the site of stenosis or fistula creates more peritracheal adhesions, and a tracheal appliance further complicates the picture. This makes the decision about how much trachea to resect difficult. In contrast, patients with tracheal tumors have focal pathology, minimal surrounding inflammation, and a history of prior procedures that has typically been limited to endobronchial ablation or tumor debridement. The caveat is that tracheal tumors treated with induction radiation may be difficult or impossible to manage surgically. If tracheal resection is an option, preoperative radiation should be assiduously avoided. The experience with TRR after radiation is small but available evidence suggests that the likelihood of an anastomotic complication increases considerably (10). Patients with ILTS represent a special group in whom the incidence of complications is relatively low. These patients tend to be healthy women with focal subglottic pathology amenable to short-segment resection. Anastomotic complications occur in this group at a rate of only 2.4%, compared to 5.3% for tumors, 11% for PTIS, and 14.3% for TEF.
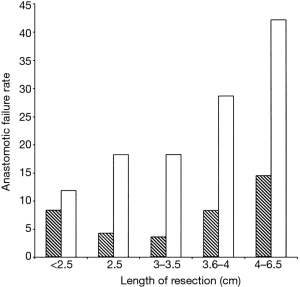
Revision tracheal surgery is particularly challenging. Donahue (11) found that of 75 patients undergoing redo TRR and LTRR, complications occurred in 39%. This finding is supported by Wright’s series, with the additional observation that the incidence of anastomotic failure increased compared to initial surgery regardless of the length of trachea resected (Figure 1). Revision TRR and LTRR is difficult because anastomotic tension is unavoidable: additional trachea must be resected and scarring from the previous operation limits the mobility of the remaining trachea. Unfortunately, many patients are not candidates for reoperation because of insufficient residual tracheal length. If reoperation is attempted, at least six months should pass from the initial operation to allow time for peritracheal inflammation to resolve.
Patients with medical comorbidities are at higher risk following surgery. In particular, diabetes has been shown to increase the incidence of anastomotic complications by an odds ratio of 2.7. Diabetes is known to impair microvascular circulation and decreases perfusion to the tracheal anastomosis. Systemic steroids also impair wound healing and should be weaned prior to resection. Preoperative steroids do not prevent airway edema and should not be used to that end. Obesity has also been shown to correlate with an increase in postoperative complications. A program of weight loss and improved glycemic control prior to TRR and LTRR is of theoretical benefit.
Optimization of the patient’s airway prior to TRR and LTRR is critical to the success of the operation. Preoperative bronchoscopy demonstrating abundant secretions or significant tracheobronchitis should prompt a course of inhaled or systemic antibiotics. Thorough airway assessment includes direct visualization of the vocal cords. Glottic pathology should be addressed prior to a subglottic or tracheal surgery. Emergent TRR or LTRR is essentially never indicated. Stenoses may be dilated or managed with an airway appliance placed at the level of planned resection. Tumors may be cored out or ablated. Self-expanding metal stents should be avoided because of their tendency to produce inflammation and granulations, effectively lengthening the amount of airway that should be resected. If a tracheostomy or t-tube is required the stoma should mature completely before proceeding with surgery. Likewise, preoperative decannulation is desirable but if achieved the stoma should be allowed to heal for several weeks. The impact of a tracheal appliance on postoperative complications manifests in several ways. Firstly, a tracheostomy or t-tube promotes colonization of the airways with pathogens that produce local inflammation and may influence healing. Secondly, a stoma placed remotely from the site of stenosis may cause malacia in that segment of trachea, or occasionally result in a second site of stenosis. Lastly, scarring from prior neck surgery limits mobility of the trachea and places tension on the anastomosis. These factors explain the observed twofold increase in complications associated with preoperative tracheostomy. It should be noted, though, that the need for postoperative tracheostomy is in of itself an independent risk factor (1).
Intraoperatively
Grillo and colleagues popularized a technique for tracheal resection that addresses several common postoperative concerns. Most tracheal pathology can be approached through a transverse cervical incision. When a stoma is present the skin around the area should be widely excised with the incision to decrease the likelihood of wound infection. Dissection is performed directly on the trachea to avoid the recurrent laryngeal nerves. No attempt is made to identify the nerves because doing so places them at risk. Circumferential dissection of the trachea is performed only at the level of pathology and carried a centimeter or so cephalad and caudad. This prevents disruption of the lateral blood supply to the trachea. Two large traction sutures decrease tension on the anastomosis, which is performed with interrupted absorbable sutures tied such that the knots are extraluminal. Grillo found that the transition from nonabsorbable (polyester) to absorbable (vicyrl) suture resulted in a dramatic decrease in the incidence of granulation tissue formation. Once constructed, the anastomosis is covered anteriorly with a well-vascularized muscle flap if the innominate artery is adjacent to the anastomosis. In patients who are at particularly high risk for anastomotic problems, such as those who have undergone preoperative radiation or prior tracheal surgery, buttressing the anastomosis with omentum is a reasonable alternative.
The degree of tension on the anastomosis is the most significant technical factor that influences the rate of postoperative anastomotic complications. In turn, tension correlates linearly with the length of trachea resected. Wright found that that for adult patients the incidence of anastomotic separation doubles when greater than 4 cm of trachea are removed. This trend is more pronounced for patients undergoing redo tracheal resection. Several maneuvers can be performed to reduce anastomotic tension. A suprahyoid release—first described by Montgomery in 1974 (12)—gains an additional 1–2 cm of length following upper tracheal resection and can be performed through a cervical incision. A pericardial release is more effective for lower tracheal or carinal resection and requires a separate thoracic incision. Release maneuvers are generally well tolerated and should be performed liberally if the surgeon deems the anastomosis to be under excessive tension. In Wright’s series, 81 patients underwent release and 21 (25%) subsequently developed an anastomotic complication. This figure does not demonstrate a causal relationship between release maneuvers and anastomotic complications, but rather supports the idea that an experienced surgeon’s assessment of intraoperative tension is quite reliable. It is probable that most or all of those 81 patients would have had an anastomotic complication in the absence of a release maneuver.
Postoperatively
Extubation in the operating room is the goal following TRR and LTRR. If postoperative anastomotic or laryngeal edema precludes immediate extubation the patient should be temporized with a small, uncuffed endotracheal tube and returned to the operating room after 24–48 hours for a second attempt. Repeated failure should be addressed with a small tracheostomy placed at least 2 cm distal to the anastomosis. Alternatively, if the surgeon judges that timely extubation is unlikely, a tracheostomy may be placed in this location at the time of initial surgery.
The neck is maintained in gentle flexion during the initial postoperative period to prevent tension on the anastomosis. This is achieved by use of a guardian suture placed between the submental crease and presternal skin to prevent hyperextension. Some centers do not use a guardian suture with compliant patients and have found that its omission decreases length of stay (2).
Retching and vomiting are potentially catastrophic events in a patient with a fresh tracheal anastomosis given the association with sudden neck hyperextension and the potential for aspiration of gastric contents. Prevention and management of postoperative nausea, both by encouraging non-narcotic pain management and with liberal use of antiemetics, is an important part of the recovery pathway.
Voice rest is helpful in preventing laryngeal edema, particularly in patients undergoing LTRR. Patients are instructed to limit speech to no more than a whisper for the first week or so, and to avoid loud speech for several weeks after that.
Swallowing dysfunction is addressed proactively. All patients should work with a speech pathologist prior to initiating a diet and a modified barium swallow should be obtained when swallowing dysfunction is suspected. Some centers obtain such studies routinely (2).
Routine bronchoscopy is performed on all patients about one week after surgery. Direct visualization of the anastomosis is the most sensitive way to assess healing and, if necessary, initiate aggressive management of subclinical anastomotic issues. A low threshold for performing bronchoscopy earlier if concerning symptoms are present is crucial in preventing an anastomotic complication from causing significant morbidity or death. Stridor, voice changes, excessive secretions, subcutaneous air, or wound infection may all be signs of an anastomotic problem and are an indication for urgent bronchoscopy.
Management of specific complications
Granulation tissue
Granulation tissue formation occurs on a spectrum ranging from mild inflammatory changes at the anastomosis to, rarely, complete airway obstruction. This complication is seen far less frequently in the era of absorbable sutures. The time course is usually days to weeks after surgery. Obstructive airway symptoms suggest the diagnosis and bronchoscopy confirms it. The majority of patients can be managed with local debridement using the tip of a rigid bronchoscope. Injection of corticosteroids around the area of granulation following debridement may be of some benefit. Endoscopic laser has been used for patients with granulation tissue from airway stents but no data exist regarding its use in the context of an airway anastomosis. When granulation tissue becomes severe and airway obstruction is a concern, insertion of a t-tube should be considered and reoperation may ultimately be required.
Restenosis
Restenosis of the trachea may be the consequence of an anastomotic problem or be part of the natural history of the patient’s underlying pathology. Patients with PITS are at highest risk for restenosis due in part to the potential for diseased trachea to be left behind at the time of initial operation. If this is the case, restenosis typically presents in the early postoperative period. For most patients, though, restenosis occurs over a period of months and is the product of tension on the anastomosis, ischemia of the cut ends of the trachea, subclinical anastomotic separation, or some combination of the above. Evaluation and dilation with flexible and rigid bronchoscopy is both diagnostic and therapeutic. Reoperation is indicated for patients in whom dilation fails to provide durable relief. A t-tube may be used to temporize patients prior to reresection or as a permanent solution for patients who lack sufficient residual trachea.
Anastomotic separation
Separation of the tracheal anastomosis may present dramatically with loss of the airway and extremis, insidiously with wound infection, subcutaneous air or progressive stridor, or asymptomatically as a finding on routine bronchoscopy. Most commonly it occurs within the first days or weeks after surgery but more remote events have been described. Excessive tension is usually at fault. The resulting defect is most often anterior where the bulk of tension is distributed. For the patient presenting in extremis the priority is stabilizing the airway. Concomitant laryngeal edema can make this a harrowing experience. Reopening the incision at bedside and cannulating the distal airway is a measure of last resort. With the airway secure, or for the patient with a more subtle presentation, the diagnostic procedure of choice is bronchoscopy performed in the operating room. If separation is confirmed the neck is explored. When the degree of separation is small it may be sufficient to cover the defect with a muscle flap. For most patients, though, insertion of an airway appliance through the defect is indicated. A t-tube is preferred if the upper airway remains patent. More commonly, anastomotic separation is accompanied by significant laryngeal edema that obstructs the upper airway and mandates placement of a tracheostomy. Immediate revision of a tracheal anastomosis after dehiscence should generally be avoided; however, for a structurally normal anastomosis that is pulled apart by sudden violent neck extension as with coughing or retching, one might reasonably attempt suture repair.
For patients with a small anastomotic defect and minimal or no symptoms it may be possible to avoid re-exploration. Antibiotic therapy with or without local drainage is instituted to control local contamination, voice rest and neck flexion are continued, and the anastomosis is allowed to heal with time. Hyperbaric oxygen therapy (HBOT) has been used to good effect in these situations. Laboratory studies performed in rats demonstrate improved tracheal healing associated with HBOT (13). Stock et al. (14) reported on five patients with minor anastomotic separation after TRR performed at Massachusetts General Hospital who were treated with HBOT (2 atmospheres for 90 minutes for an average of 13 sessions). All were able to avoid subsequent revision of the anastomosis over a follow-up period of 1 to 3 years. One patient required debridement for granulation tissue and another required tympanostomy tubes to tolerate HBOT. Further studies are needed but sufficient evidence exists to support the use of HBOT for selected patients with minor anastomotic complications.
Wound infection
Cervical wound infections occur rarely but must be promptly diagnosed and treated—both to prevent erosion into the anterior anastomotic area and in case the infection is the first sign of a brewing anastomotic problem. Redness, drainage and increased incisional pain suggest a wound infection. Cough, increased sputum production, or stridor associated with a wound infection is suspicious for an underlying anastomotic. Management consists of opening and culturing the wound. A CT scan is done to look for extraluminal air, undrained collections and other signs suggestive of an anastomotic problem. If there is a reasonable chance of an anastomotic problem, urgent bronchoscopy should be done. If there is a partial anterior dehiscence associated with a wound infection it may be addressed by placing a small temporary tracheostomy and properly draining the surrounding infection. Most patients will heal quickly without need for major revision.
TIF and TEF
Fistula formation between the anastomosis and the innominate artery is often a fatal event. Two of the three patients who developed TIF in Wright’s series subsequently died. TIF forms as a result of anterior separation of the anastomosis causing soft tissue inflammation and infection that in turn erodes into the innominate artery. Dissection around the artery at the time of resection exposes it and places it at risk for involvement in a fistula should anastomotic separation occur. Hemoptysis is the cardinal sign of TIF. A sentinel, small-volume episode of hemoptysis may precede a life-threatening event. Airway symptoms suggestive of anastomotic separation are typically present. Small-volume hemoptysis in a stable patient may be investigated with a CT-angiogram prior to bronchoscopy. Massive hemoptysis mandates immediate return to the operating room. Exposure of the innominate artery requires a partial or complete sternotomy. The artery is ligated and divided and the cut ends are separated from the infected field with healthy tissue. The airway defect is then addressed in the same fashion as described above. Dividing the artery should not result in cerebral ischemia provided that the bifurcation of the right subclavian and carotid arteries remains intact.
Fistula from the anastomosis to the esophagus is also rare but can be a significant source of morbidity. TEF occurs in the context of a posterior separation of the anastomosis. The esophagus becomes involved either through direct injury during tracheal dissection or secondarily from the resulting infection. Patients may present months after surgery with post-prandial cough, pneumonia, or obstructive upper airway symptoms. Barium swallow and bronchoscopy confirm the diagnosis. TEF is managed in delayed fashion. The airway is controlled with a tracheostomy or t-tube and the patient is fed enterally until operative conditions improve. Definitive management includes revision of the airway anastomosis, two-layer repair of the esophageal defect and interposition of healthy tissue. Occasionally it may be sufficient to repair the esophagus alone and allow a small defect in the airway to heal over time. In Wright’s series, all three patients who developed TEF ultimately had a good outcome.
Laryngeal edema
Laryngeal edema causing obstructive airway symptoms is more common following laryngeal resection. For high laryngeal resection postoperative swelling is the rule. Patients present with voice changes described as “husky”. Stridor is sometimes present as well. Bronchoscopy is usually diagnostic and importantly rules out concomitant anastomotic separation. Mild cases are addressed with steroids, diuretics, nebulized epinephrine, and head elevation. If loss of the airway is a concern the patient should be intubated with a small, uncuffed endotracheal tube. Edema that fails to resolve after a few days of intubation and medical therapy is an indication for tracheostomy.
Recurrent laryngeal nerve palsy
Recurrent laryngeal nerve injury following TRR and LTRR is rare if proper technique is observed and the nerves are kept from entering the surgical field. However, some degree of postoperative hoarseness is relatively common and may be the result of mild laryngeal edema or transient nerve palsy brought on by inflammation or traction. Hoarseness should be investigated with direct laryngoscopy but even if immobility of one of the vocal cords is demonstrated the potential for recovery cannot be known. Given this ambiguity, at least six months should transpire before medialization laryngoplasty is considered. Temporary injection of the cord is indicated if there is objective evidence of ongoing aspiration. In the interim, patients with suspected nerve injury should work closely with a speech pathologist to improve swallowing and phonation.
Swallowing dysfunction
All laryngeal resections produce some degree of edema that contributes to postoperative swallowing dysfunction. Additionally, a suprahyoid release impairs normal motion of the larynx during swallowing and extended trachea resection may do the same by tethering the larynx and preventing normal elevation. Some component of recurrent laryngeal nerve palsy may be at play for some patients. Older patients tolerate modest swallowing dysfunction poorly. Fortunately, improvement almost always occurs with time and dedicated work with a speech pathologist. Rarely patients require placement of a gastrostomy tube to maintain nutritional intake during this period.
Outcome after an anastomotic complication
Even in the presence of an anastomotic complication results following TRR and LTRR are good. Of the 81 patients who developed an anastomotic complication in Wright’s series (37 separation, 37 restenosis, 7 granulation tissue formation), 41 (51%) ultimately had a satisfactory airway. Multiple dilations and temporary airway appliances were necessary in 2 and 23 patients, respectively. Sixteen patients required reoperation but all ended up with a satisfactory result. The remaining 40 patients progressed to needing a permanent tracheostomy (14 patients), t-tube (20 patients) or died as a result of the anastomotic complication. Three patients died from anoxic injury, two from TIF, and one from mediastinitis. Death in the absence of an anastomotic complication occurred in only five patients (0.6%). An anastomotic complication was associated with a thirteen-fold increase in mortality. Median hospital stay was also extended, from 8 days without an anastomotic complication to 14 days with one.
Conclusions
Complications following tracheal surgery are not common but must be addressed promptly and effectively when they arise to prevent major morbidity. Stabilization of the patient’s airway, evaluation with bronchoscopy, and use of a temporizing airway appliance are all important components of the management of airway complications. Most patients with an anastomotic complication respond well to aggressive management and will go on to have a good outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wright CD, Grillo HC, Wain JC, et al. Anastomotic complications after tracheal resection: prognostic factors and management. J Thorac Cardiovasc Surg 2004;128:731-9. [PubMed]
- Mutrie CJ, Eldaif SM, Rutledge CW, et al. Cervical tracheal resection: new lessons learned. Ann Thorac Surg 2011;91:1101-6; discussion 1106. [PubMed]
- Bibas BJ, Terra RM, Oliveira Junior AL, et al. Predictors for postoperative complications after tracheal resection. Ann Thorac Surg 2014;98:277-82. [PubMed]
- Piazza C, Del Bon F, Paderno A, et al. Complications after tracheal and cricotracheal resection and anastomosis for inflammatory and neoplastic stenoses. Ann Otol Rhinol Laryngol 2014;123:798-804. [PubMed]
- Couraud L, Bruneteau A, Martigne C, et al. Prevention and treatment of complications and sequelae of tracheal resection anastomosis. Int Surg 1982;67:235-9. [PubMed]
- Grillo HC, Zannini P, Michelassi F. Complications of tracheal reconstruction. Incidence, treatment, and prevention. J Thorac Cardiovasc Surg 1986;91:322-8. [PubMed]
- Macchiarini P, Verhoye JP, Chapelier A, et al. Partial cricoidectomy with primary thyrotracheal anastomosis for postintubation subglottic stenosis. J Thorac Cardiovasc Surg 2001;121:68-76. [PubMed]
- Grillo HC, Donahue DM, Mathisen DJ, et al. Postintubation tracheal stenosis. Treatment and results. J Thorac Cardiovasc Surg 1995;109:486-92; discussion 492-3. [PubMed]
- Wright CD, Graham BB, Grillo HC, et al. Pediatric tracheal surgery. Ann Thorac Surg 2002;74:308-13; discussion 314. [PubMed]
- Muehrcke DD, Grillo HC, Mathisen DJ. Reconstructive airway operation after irradiation. Ann Thorac Surg 1995;59:14-8. [PubMed]
- Donahue DM, Grillo HC, Wain JC, et al. Reoperative tracheal resection and reconstruction for unsuccessful repair of postintubation stenosis. J Thorac Cardiovasc Surg 1997;114:934-8; discussion 938-9. [PubMed]
- Montgomery WW. Suprahyoid release for tracheal anastomosis. Arch Otolaryngol 1974;99:255-60. [PubMed]
- Gorur R, Hahoglu A, Uzun G, et al. Effects of hyperbaric oxygen therapy on wound healing after tracheal resection and end-to-end anastomoses in rats: results of early observations. Thorac Cardiovasc Surg 2008;56:359-62. [PubMed]
- Stock C, Gukasyan N, Muniappan A, et al. Hyperbaric oxygen therapy for the treatment of anastomotic complications after tracheal resection and reconstruction. J Thorac Cardiovasc Surg 2014;147:1030-5. [PubMed]


