Effectiveness of hemostasis with Foley catheter after vacuum-assisted breast biopsy
Introduction
Breast benign diseases have happen more frequently than breast cancer. Estimates are that 60% of all women will experience this condition (1). The most common benign breast diseases are fibroadenoma and fibrocystic disease. It therefore represents a significant part of a breast surgeon’s workload. Though these diseases are not life threatening, these patients are very anxious about their condition and need to be managed in the setting of a multidisciplinary team (2).
There is controversy over how to treat a suspected breast benign lesion. The key to evaluation of breast benign lesions is triple assessment with careful examination; appropriate imaging to visualize an abnormality in the breast at all ages and core biopsy where appropriate. Fine-needle aspiration (FNA) and core-needle biopsy (CNB) are well-established, valuable techniques that are still used in most cases, whereas vacuum-assisted breast biopsy (VABB) is a more recent technique (3).
Introduced in the late 1990s, the VABB has become a major tool for evaluating breast benign lesions (4). VABB has proven clinical value and can be used under mammographic, sonographic, and magnetic resonance imaging guidance (5). The effectiveness and high accuracy of non-operative diagnosis achieved by VABB has led to reduce numbers of open surgical biopsies (6). Despite an effective, increasingly used method for the diagnosis of nonpalpable breast lesions, VABB has inevitable complications—bleeding and hematoma at a rate of about ten percent (7). However, no systematic efforts have been undertaken to limit or prevent hematoma formation. Zografos et al. reported that VABB-induced hematoma might be achieved via the insertion of a thin intravascular catheter (e.g., a Fogarty catheter) adjacent to the VABB probe (8). Later, Zografos et al. reported a case that using of Fogarty catheter to limit hemorrhage and hematoma after VABB (9).
To limit or reduce the rates of interventional bleeding and post-interventional hematoma after VABB, in this study we evaluated the hemostasis effects of the Foley catheter. Our hospital achieved hemostasis by using a Foley catheter method for internal hemostasis reducing the rates of post-operative bleeding and hematoma. In contrast, more interventional bleeding and post-interventional hematoma were detected when external compression hemostasis was used.
Patients and methods
Patients
A total of 437 ultrasound-guided VABB using the Mammotome biopsy system were performed in 282 patients who were all female. They were admitted to the Obstetrics and Gynecology Hospital of Fudan University Breast Surgery Outpatient Department. These patients enrolled in the randomized prospective control study during the period of June 2012 to October 2013. All the women were required to sign the informed consent document prior to study entry.
Inclusion criteria
After undergoing the physical examination, the patients received a B-ultrasound examination. Women 40 years of age or older were required to provide a recent (within the previous 6 months) mammogram. All of these reports needed to be BI-RADS category 3, ruling out malignant diseases.
Exclusion criteria
Women with bleeding disorders, those taking anticoagulants or other medications that could contribute to bleeding abnormalities which could not be reversed, women with breast implants, and nursing or pregnant women were considered ineligible for study participation. Those with uncontrolled diabetes or high blood pressure were excluded.
Operation procedure
Ultrasound guidance was performed using equipment having a minimum fixed frequency of 7 MHz or variable frequencies with a range of 7 to 10 MHz. The site of insertion was marked on the skin by an oily marker. Local anesthesia was selected. Anesthetics were 0.5% lidocaine with 1:500,000 adrenaline. After administration of local anesthetic, we set the Mammotome system to the “position” mode, and pushed the 8-gauge probe into the breast through a 3 mm skin incision. The probe was guided into biopsy position under direct ultrasound visualization. Multiple core samples were taken until the mass was completely removed as determined by real-time ultrasound imaging of the biopsy site. The procedure was performed by the same team of two surgeons with at least 3 years of specialized cumulative experience in VABB that was conducted in more than 300 patients every year.
Random allocation
Patients were randomized to one of either study group or control group. In the study group, a 12B Foley catheter with a 10 mL balloon through the same breast parenchymal track into the excision cavity under real-time sonography guidance. The balloon was inflated with 5 to 10 mL of normal saline according to the size of the excision cavity for 5-10 minutes. Then the normal saline was discharged by a syringe and the Foley catheter was extracted (Figures 1,2). In the control group, manual compression to the breast was performed for 5-10 minutes to assure adequate hemostasis. Finally, the 3 mm incision was covered with a sterile plaster and a thorax pressure bandage. Histological examination was performed on all specimens. All patients underwent clinical and ultrasound assessment of the biopsied area immediately after surgery and 1 week later.
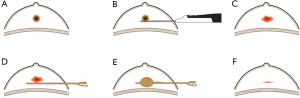
Definitions
Bleeding during intervention was decided by more than 20 mL blood aspirated. Hematoma post intervention was defined as the extension of more than 2 cm × 2 cm × 2 cm in projection of the target area in post-interventional sonography. The time of procedure was counted from the condution of local anesthetic to the skin incision covered with a sterile plaster.
Statistics
The software program SPSS 17.0 for Windows was used for all statistical analyses. For univariate comparisons of categorical variables, a Chi-Square test was utilized. Continuous variables were expressed as median (range). For univariate comparisons of continuous variables, an independent-samples t-test was utilized. A P value of <0.05 was considered to be significant.
Results
A total of 437 consecutive 8-gauge ultrasound-guided VABB procedures were performed in 282 patients with a median age of 33.3 years (range, 16-58 years). Patient demographics and characteristics of the original breast lesion are shown in Table 1. The histopathological examinations of the 437 lesions extirpated revealed that all of the lesions were benign: 333 fibroadenomas (76.2%), 86 fibrocystic lesions (19.7%), 3 papillomas (0.7%), 10 galactocele (2.3%), 5 chronic galactophoritis (1.1%).

Full table
Bleeding and hematoma were detected more often after compression was used to achieve hemostasis compared with the Foley catheter method. The mean time of every VABB procedure in which a Foley catheter was significantly less than when compression was used (Table 2). Comparing the bleeding rates for hemostasis induced using a Foley catheter vs. hemostasis induced using compression (Figure 3A,B); the difference reached a significant level in the cases of multiple lesions (Figure 3A). Hematoma occurred significantly more often when external compression was used compared to when a Foley catheter was used, both in single lesions and multiple lesions (Figure 4A). When lesions ≥15 mm were excised, the rates of interventional bleeding and post-interventional hematoma were significantly lower in the study group, where the Foley catheter was used (Figure 3B, Figure 4B). In the inter-group analysis of bleeding rates and hematoma rates in the Foley catheter compression groups in upper external quadrant and other quadrants, there was significantly less bleeding and less hematoma when using the Foley catheter in upper external quadrant (Figure 5). No intraoperative surgical management of any post-procedural complications was ever required. No post-procedural infectious complication was encountered. Besides, no patients using hemostasis with Foley catheter complained breast pain or discomfort under the situation of local anesthetic.
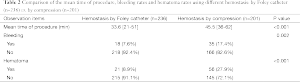
Full table



Discussion
In the last decade minimal invasive complete excision of benign breast tumors has rapidly gained acceptance as the technique of alternative to open surgery (10). However, the presence of interventional bleeding and post-interventional hematoma was the most common complication following VABB (11). Traditionally, only manual compression to the breast was performed after VABB. Dahabreh et al. reported the results of eight studies on ultrasound guided vacuum assisted biopsies with a median reported hematoma rate of 13% (range, 3.7-23%) and a bleeding rate of 2.5% (range, 1-4.9%) (12). Schaefer et al. reported significantly more bleedings and post-interventional hematomas for 8-gauge-Mammotome®-system vs. 11-gauge-Mammotome®-system (41.9% vs. 8.4%, P<0.001; 35.5% vs. 16.7%, P=0.029), no significant-differences for the ATECR-systems 9-gauge vs. 12-gauge (26.9% vs. 29.7%, P=0.799; 42.3% vs. 43.2%, P=0.596) (13).
Zagouri et al. reported that clinically significant and subsequently organized hematomas were significantly more frequent in the extended protocol than in the standard protocol (7.5% vs. 3.5%, P=0.038) (14). Zagouri et al. also reported the likelihood of hematoma is increasing along with increasing amount of blood suctioned, reaching a plateau approximately at 80 cc of blood lost (15). Zografos et al. reported that Elevated IL-6 at 1 hour after the end of VABB might point to subsequent organization of the hematoma and the need for appropriate action (16). Fischman et al. first reported a case of transcatheter embolization of uncontrolled breast hemorrhage after VABB (17). Additional, Sun et al. suggested that in the face of significant intractable bleeding, a heightened awareness of the possible need for surgical intervention should be maintained (18). Additional, Melotti et al. reported that discontinuing anticoagulation medication before core needle breast biopsy may be unnecessary when the need for biopsy is urgent (7). In our early work, there were some cases need open operation because of persistent bleeding. But this serious complication had not appeared in this study. There are some reports that hematoma may lead to swilling pain, secondary infection. Obviously, this complication will lead to prolonged treatment time, increased treatment costs, increase the suffering of patients. In our study, there were no serious complications that had to be conducted by surgical procedure. But the hemostasis method by Foley catheter reduced the rates of post-operative bleeding and hematoma comparing to that by compression. Moreover, during the 3 weeks, we followed up the 60 patients who suffered breast swilling pain after VABB because of the post-intervention hematoma. According to the pain analogue scale (19), the score was above 25 requiring analgesics for relief in 2 out of 15 (13.3%) patients who used Foley catheter procedure comparing that in 31 out of 45 (68.9%) patients who used external compression (P<0.001). In a sense, the procedure using Foley catheter can relive the swilling pain caused by the post-intervention hematoma and reduce the rate of taking medicine.
In 2008, Zografos et al. had indicated that Fogarty catheter seemed technically feasible to limit or prevent hemorrhage and hematoma after VABB (8,9). While to the best of our knowledge, there is currently no other literatures evaluating the effectiveness of hemostasis induced using Foley catheter after VABB. In the current study, the interventional bleeding rate was obviously lower when hemostasis was achieved using a Foley catheter vs. with manual compression (P=0.002). The stratification analysis revealed significantly less bleeding for the Foley catheter method in the cases of multiple lesions (P=0.018), in lesions ≥15 mm (P=0.034) and in the group of lesions in the upper external quadrant (P=0.004). These results can be explained by the fully inflation of the Foley catheter balloon in the excision cavity to assure adequate hemostasis. Moreover, the position of the inflated balloon was fixed and the more precise hemostasis position was not changed. However, in the cases of single lesion (P=0.346), in the group of lesions <15 mm (P=0.531) and in the group of lesions in the other quadrants (P=0.130), the bleeding rates of hemostasis by Foley catheter and by compression did not differ significantly. These similar bleeding rates obviously occurred in single, smaller lesions in the quadrants excluding the upper external quadrant.
Likewise, the post-interventional hematoma rate was significantly lower in the Foley catheter group compared with the control group (P<0.001). The stratification analysis revealed significantly less hematoma for the Foley catheter method in the cases of single (P=0.017) and multiple (P=0.020) lesions, in lesions ≥15 mm (P<0.001) and in the group of lesions in the upper external quadrant (P<0.001). However, in the group of lesions <15 mm (P=0.340) and in the group of lesions in other quadrants (P=0.094), the bleeding rates did not differ significantly between hemostasis using a Foley catheter and hemostasis using compression. These similar bleeding rates obviously occurred in smaller lesions in the quadrants excluding the upper external quadrant.
Therefore, the methods of hemostasis can be chosen according to the number, the size and the location of lesions. When more numerous and larger lesions in the upper external quadrant are excised using VABB, then hemostasis using a Foley catheter can be selected. In this way, the occurrence of bleeding and hematoma will be reduced.
In our study, the mean time of every excision procedure followed by either hemostasis using a Foley catheter or hemostasis using compression was 33.6 and 45.5 minutes, respectively. It can save about 10 minutes or so if the Foley catheter method is chosen. It is our opinion that when hemostasis by Foley catheter is carried out after VABB, we can excise the next lesion at the same time without waiting for adequate hemostasis to be detected as is necessary in external compression. In this regard, especially in the operative procedure of the bilateral lesions, we can conduct the excision of the lateral lesions after the other lateral cavity has been tamponaded with the inflated Foley catheter. The manual compression is the traditional hemostasis after VABB. In clinical, we observed the local skin pitting at the excision cavity location after external compression hemostasis or the thorax bandage pressure, which interfered with the cosmetic outcome. Moreover, the translocation of thorax pressure bandage may cause pressure to be put the wrong location, sometimes causing post-interventional bleeding or hematoma. However, hemostasis using a Foley catheter can avoid the above disadvantages. In addition, no post-procedural infectious complication was encountered. Thus the method of Foley catheter can be considered safe.
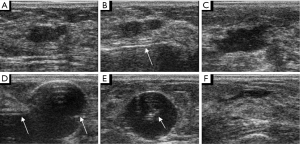
Sometimes, when the breast parenchymal track is not straight, it is difficult to place the Foley catheter into the excision cavity because it is too soft (Figure 6A). In our study, this situation happened in 2 out of 18 (11.1%) bleeding lesions with Foley catheter hemostasis. In another condition, the interventional bleeding or the post-interventional hematoma was produced from the breast parenchymal track or from the point of needle but not from the excision cavity. This incidence is 5 out of 18 (27.8%). A limitation of the study is that we cannot manage these situations using the Foley catheter method. So it is essential that we improve the design of the Foley catheter in the further studies. For example, we can improve the device by putting a stick into the Foley catheter (Figure 6B). So, it is hard enough to go through the track and reach the excision cavity. In addition, we can design a new catheter with three balloons tamponaded in the positions of the point of needle, the excision cavity and the breast parenchymal track, respectively (Figure 6C).

Conclusions
In summary, hemostasis using a Foley catheter after VABB is very effective and safe, providing an appropriate alternative for hemostasis using compression. In this regard, we believe that the Foley catheter method should be offered to appropriate patients who accept the therapy of VABB.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: H Song.
References
- Fibrocystic breast changes. MedlinePlus. U.S. National Library of Medicine. Updated 05 Nov 2012. Accessed 10 Apr 2002. Available online: http://www.nlm.nih.gov/medlineplus/ency/article/000912.htm
- Datta S, Davies EL. Benign breast disease. Surgery 2013;31:22-6.
- Lee SH, Kim EK, Kim MJ, et al. Vacuum-assisted breast biopsy under ultrasonographic guidance: analysis of a 10-year experience. Ultrasonography 2014;33:259-66. [PubMed]
- Bear HD. Image-guided breast biopsy--how, when, and by whom? J Surg Oncol 1998;67:1-5. [PubMed]
- Park HL, Hong J. Vacuum-assisted breast biopsy for breast cancer. Gland Surg 2014;3:120-7. [PubMed]
- Ding B, Chen D, Li X, et al. Meta analysis of efficacy and safety between Mammotome vacuum-assisted breast biopsy and open excision for benign breast tumor. Gland Surg 2013;2:69-79. [PubMed]
- Melotti MK, Berg WA. Core needle breast biopsy in patients undergoing anticoagulation therapy: preliminary results. AJR Am J Roentgenol 2000;174:245-9. [PubMed]
- Zografos GC, Zagouri F, Sergentanis TN, et al. Hematoma after vacuum-assisted breast biopsy: a preventable condition? Acta Radiol 2008;49:277; reply 277. [PubMed]
- Zografos GC, Zagouri F, Sergentanis TN, et al. Use of fogarty catheter to limit hemorrhage and hematoma after vacuum-assisted breast biopsy. Acta Radiol 2008;49:752-4. [PubMed]
- Eller A, Janka R, Lux M, et al. Stereotactic vacuum-assisted breast biopsy (VABB)--a patients' survey. Anticancer Res 2014;34:3831-7. [PubMed]
- Al-Harethee W, Theodoropoulos G, Filippakis GM, et al. Complications of percutaneous stereotactic vacuum assisted breast biopsy system utilizing radio frequency. Eur J Radiol 2013;82:623-6. [PubMed]
- Dahabreh IJ, Wieland LS, Adam GP, et al. Core Needle and Open Surgical Biopsy for Diagnosis of Breast Lesions: An Update to the 2009 Report [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014 Sep. Report No.: 14-EHC040-EF. AHRQ Comparative Effectiveness Reviews. 2014;
- Schaefer FK, Order BM, Eckmann-Scholz C, et al. Interventional bleeding, hematoma and scar-formation after vacuum-biopsy under stereotactic guidance: Mammotome(®)-system 11 g/8 g vs. ATEC(®)-system 12 g/9 g. Eur J Radiol 2012;81:e739-45. [PubMed]
- Zagouri F, Gounaris A, Liakou P, et al. Vacuum-assisted breast biopsy: more cores, more hematomas? In Vivo 2011;25:703-5. [PubMed]
- Zagouri F, Sergentanis TN, Domeyer P, et al. Volume of blood suctioned during vacuum-assisted breast biopsy predicts later hematoma formation. BMC Res Notes 2010;3:70. [PubMed]
- Zografos GC, Zagouri F, Sergentanis TN, et al. Hematoma after vacuum-assisted breast biopsy: are interleukins predictors? Onkologie 2009;32:395-7. [PubMed]
- Fischman AM, Epelboym Y, Siegelbaum RH, et al. Emergent embolization of arterial bleeding after vacuum-assisted breast biopsy. Cardiovasc Intervent Radiol 2012;35:194-7. [PubMed]
- Sun S, Hennessey H, Kam Nakch I, et al. Compression-refractory breast hematoma secondary to pseudoaneurysm after stereotactically guided vacuum-assisted biopsy: the critical role of urgent surgical evacuation. J Clin Ultrasound 2014;42:492-4. [PubMed]
- Ehrmann EH, Messer HH, Adams GG. The relationship of intracanal medicaments to postoperative pain in endodontics. Int Endod J 2003;36:868-75. [PubMed]


