Pulmonary benign metastasizing leiomyoma: a case report and literature review
Introduction
Benign metastasizing leiomyoma (BML) is a rare condition that occurs in all age groups, mostly between age 30 and 74, and is particularly prevalent among women of late childbearing age. Its clinical course is closely associated with sex hormone levels. All BML patients have a history of uterine leiomyoma and/or myomectomy. Symptoms usually develop and a diagnosis is made within 15 years (1), and BML is often associated with distant metastases from the uterus, such as benign leiomyoma in the lungs, paraaortic lymph nodes, abdominal lymph nodes, heart, breasts, liver, esophagus, trachea, limb striated muscles, skeletal muscle, skin, scars and central nervous system (2-7). The lungs are the most common site of metastasis, with characteristic scattered nodules (8-12). The pathological results of a pulmonary nodule examination usually indicate a specific smooth muscle phenotype with a low proliferation index and slow growth. The nodules are also positive for the estrogen receptor (ER) and the progesterone receptor (PR), revealing its uterine origin (13). This type of disease is usually called pulmonary benign metastasizing leiomyoma (PBML).
Since the first case of metastasizing leiomyoma was discovered and reported by Steiner (14) in 1939, more than 100 cases of PBML have been reported. Most cases have been discovered by chest X-ray or CT scan during routine examinations. Some patients have symptoms such as cough, dyspnea or chest pain; PBML is quite difficult to diagnose by simple medical imaging or physical examination and is often misdiagnosed as pneumonia, bronchitis, phthisic or metastasizing lung cancer. Open lung biopsy (OLB) is the standard diagnostic procedure for PBML.
We reported a case of PBML. The pulmonary metastasis appeared only 1 month after the myomectomy, which was performed earlier than in any other cases. The clinical pathology, imaging features, bronchoscopy results, immunohistochemical staining features, diagnosis, treatment and prognosis are analyzed and discussed in this article, and the literature is reviewed.
Case report
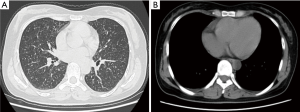
A 32-year-old non-smoking woman (BMI 26) with a history of blood transfusion was hospitalized with chest tightness and labored breathing for one month. The patient suffered from chest tightness, labored breathing and weakness after a myomectomy that had been performed one month earlier, but there was no patent cough or expectoration. The patient gave birth to a child at 30 years of age. The first menstruation of the patient was at 12 years of age and was regular thereafter, occurring every 27-28 days and lasting 4-5 days. The chest CT revealed diffuse pulmonary lesions. The laboratory examinations were positive for PPD, negative for serum anti-tuberculosis antibodies, and negative for T-spot; the blood sedimentation rate was 20 mm/h, and C-reactive protein was within the normal range. The results of a routine blood test, routine stool test, routine urine test, ESR, blood coagulation function, and myocardial enzyme determination were all within normal limits. Serum concentrations of cancer markers such as CA125, CA199, CA153 and CA242 were all within normal ranges. Carcinoembryonic antigen (CEA), neuron specific enolase (NSE) and cytokeratin 19 fragments (CYFRA21-1) were also all within normal limits. An abdominal MRI showed a hemangioma over the posterior segment of the liver and a cyst over the anterior segment of the liver. The results of a bronchoscopy were normal, and a TBLB was negative. The results of an ECT bone scan were normal. The lung function tests were within the normal limits except for a slightly low residual volume. The diffusion lung capacity was normal. Deep venous ultrasonography of the lower limbs showed no thrombosis. The patient felt no improvement taking isoniazid, rifampicin and ethambutol associated with levofloxacin antituberculosis treatment for 4 weeks. Another chest CT (Figure 1) showed a diffuse miliary shadow in both lungs, small multiple lymph nodes in the mediastinum and bilateral axillaries. Subsequently, a lung biopsy was taken by thoracoscopic surgery, which showed obvious nodules in the right lung. Multiple tissues were taken for biopsy using the thoracoscope.
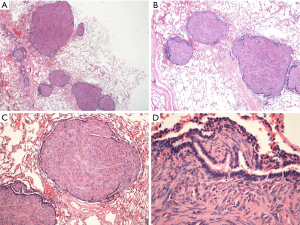
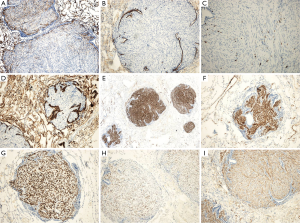
H&E staining (Figure 2) showed spindle-shaped smooth muscle tumor cells of different differentiations. The tumors appeared multi-nodular and isolated. The realm was clear, the nodular surface was coated with alveolar epithelium, some nodules had a hardened area, and the cell morphology was consistent without atypia and with rare mitoses. Immunohistochemical staining is shown in Figure 3; CD10 (Figure 3A) is the key to the differential diagnosis; we were able to make a differential diagnosis of PBML. Solitary fibrous tumors and endometrial stromal sarcoma, which are both spindle cell tumors, were evident in the lung. CD10 expression was positive in the latter two, and the CD10 expression in the PBML was negative. The negative expression of CD10 confirmed the diagnosis of PBML. Positivity for SP-B (Figure 3B) indicates a primary lung tumor, and negativity for SP-B (as in Figure 3B) is related to lung metastases. The low proliferative activity of Ki-67 [Figure 3C; Ki-67(+) <5%] showed a low tumor cell proliferation index. CK (Figure 3D) is an epithelial marker, so the PBML was inevitably negative. Positivity for α-smooth muscle actin (SMA) (Figure 3I) and desmin (Figure 3E,F) suggested that the tumor was derived from smooth muscle. Strong positivity for the ER and the PR indicated that the tumor cells originated in the uterus and were regulated by the ER and PR (Figure 3G,H). Based on all of these results, the patient was diagnosed with PBML.
The patient was given the anti-estrogen tamoxifen for 3 months. No further development of the disease or distant metastasis was found. The patient has been followed for 4 months and will continue to be followed.
Discussion
Hysteromyoma is a common gynecologic tumor, 50% of which occurs in women over 30 years of age (15). Most hysteromyoma is benign, and malignant cases account for 0.13-6% (15). PBML usually refers to benign hysteromyoma metastasizing to the lung (1,7-10,16,17), similar to distant endometriosis (13).
There are several main hypotheses for the pathological origin of PBML (18-20) to be confirmed: (I) it is derived from low-grade leiomyomatosis; (II) it is derived from benign metastasizing hysteromyoma; or (III) it is derived from a multicenter-growing leiomyomatosis. Additionally, some researchers hypothesize that BML is related to angioleiomyoma (13). The main pathological PBML lesions show no significant differences from leiomyomatosis in other parts of the body. The characteristic findings are well-defined nodules varying from miliary size to 10 cm, interwoven with a pale solid ductile cut surface with no hemorrhage or necrosis. Immunohistochemical staining of tumor issues in the lung showed that the tumor cells are characteristic of smooth muscle cell differentiation (α-SMA+, Desmin+), and the low expression of Ki-67 indicates a low tumor cell proliferation index, which indicates that the likelihood of malignancy was not high.
PBML is generally considered a monoclonal benign tumor that is potentially metastatic (21). It is hormone dependent, and its growth mainly depends on estrogen and progesterone. Estrogen causes the tumor to progress, whereas progesterone causes the tumor to subside. The tumor subsides when the patient is pregnant or menopausal. However, its pathogenesis remains unknown (21). According to a popular theory, the tumor is believed to hematogenously spread. When uterine surgery is undertaken, including uterine curettage, hysteromyomectomy and hysterectomy, the possibility of hematogenous spread by surgical induction increases and the tumor may disseminate to the lung through the venous circulation (7). However, this theory cannot explain how the lung nodules exist before a hysterectomy in some cases (22). There were miliary changes in both of the patient’s lungs only one month after the hysteromyomectomy, which suggested hematogenous metastasis to the lung. However, there was no obvious relation between the severity of the lesions and the influences of the disease on the lung. The lung function of the patient was not significantly affected even though there were miliary changes in the lung.
Because PBML progresses slowly, it is often discovered accidently during physical examination. It has a small influence on lung function. Some patients present with fever, chest stuffiness, pant, dry cough and hemoptysis, whereas most patients have no symptoms. Only when the tumor grows larger or multiplies will some patients have symptoms such as hemoptysis and chest pain. As the disease progresses, the patient may die of respiratory failure.
The plain chest film, chest CT and/or CT-PET scan of the PBML patients show several multiple or solitary shadows of different sizes that are noncalcified and with smooth edges or round lobular or round soft tissues, either well-circumscribed or cavitary, and with small quantities of pleural effusion or mild thickening of the pleura (23). Some patients have lungs densely covered by miliary nodules, with narrow or blocked tracheas and bronchi. However, there are normally no influences on the endobronchus and pleura and no mediastinal lymphadenectasis (24). Horstmann et al. (19) conducted a retrospective study of the imaging findings of 23 PBML cases. The most common features (16 cases, 70%) were multiple nodules in both lungs with smooth edges that were either lobulated or cavitary. There were also one-sided multiple lumps (4 cases, 17%) and solitary lumps (3 cases, 13%). The rare features were diffuse miliary lesions. The chest CT scan of the patient showed small miliary nodules with slight mediastinal lymphadenectasis.
It is indispensable to identify PBML from multiple pulmonary nodules or lump diseases such as benign or malignant tumor, pulmonary lymphangioleiomyomatosis, tuberculoma, sarcoidosis, pneumoconiosis, lung collagen vascular disease, inflammatory pseudotumor, metastatic carcinoma of the lung and so on. Because the imaging features of PBML are nonspecific, it is quite difficult to diagnose and differentiate. Only when the malignancy is excluded can the dissemination and metastasis of a benign tumor be confirmed (25,26).
The OLB/thoracoscopic lung biopsy is indispensable and the standard diagnosis for PBML. The OLB or thoracoscopic lung biopsy cannot be replaced with the percutaneous lung puncture biopsy. Women of childbearing age with a history of uterine myoma, especially uterine tumor surgery, should be considered for the possible diagnosis of PBML when pulmonary nodules and diffuse lesions are present.
There are several treatment options for PBML. The first choice is excision by surgery to remove the foci (27). The patients should be followed after surgery to observe closely if a new focus develops. The second option includes hysterectomy, bilateral adnexectomy and long-term hormone therapy, which blocks the hormone release to stabilize the pulmonary lesions (28). The third option is inferior vena cava filter implantation, which has a potent effect on preventing primary lesion metastasis to the lungs (26). The patients should be followed to observe closely the sizes and numbers of tumors with a thoracoscopic tumor resection or partial pulmonary lobectomy.
Because PBML normally expresses the ERs and PRs, it is crucial to control estrogen levels. Although bilateral adnexectomy has an obvious effect on tumor growth control, drug treatment has the advantages of minimal trauma and reversibility, with oral drugs relieving symptoms of patients who cannot have surgery. It is reported (6,7,13,19,29,30) that hormone therapy, such as tamoxifen, raloxifene and so on, is effective. It is largely reported that controlling estrogen levels is effective in controlling tumor growth and providing a good prognosis. The overall survival period is 6-101 months, and the median is 94 months (23). There were both cases of spontaneous recovery and cases of death (7).
To summarize, although PBML is a rarely reported disease, women of childbearing age with a history of uterine myoma, especially uterine tumor operation, should be considered for the possible diagnosis of PBML when pulmonary nodules and diffuse lesions are present. Surgery is the first choice for patients who can tolerate the operation; a biopsy should be taken for multiple lesions to identify the pathological types and sources as soon as possible. If surgery is not a good choice, anti-hormone therapy may be the only effective treatment option, and we should monitor the sex hormone levels and the relapse or distant metastasis of PBML. In our case, the patient’s imaging findings were not typical: the HRCT showed numerous miliary nodules in diffuse and random distributions in both lungs, which complicated our diagnosis. In the case reports, the times at which metastasizing leiomyoma appeared in the lung after a hysteromyomectomy were between several months and 15 years (1); however, the time to appearance of the metastasizing leiomyoma in the lung after the hysteromyomectomy was only 1 month. This result may indicate that PBML can also develop in a short period of time.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kayser K, Zink S, Schneider T, et al. Benign metastasizing leiomyoma of the uterus: documentation of clinical, immunohistochemical and lectin-histochemical data of ten cases. Virchows Arch 2000;437:284-92. [PubMed]
- Kwon YI, Kim TH, Sohn JW, et al. Benign pulmonary metastasizing leiomvomatosis: case report and a review of the literature. Korean J Intern Med 2006;21:173-7. [PubMed]
- Jo JH, Lee JH, Kim DC, et al. A case of benign metastasizing leiomyoma with multiple metastasis to the soft tissue, skeletal muscle, lung and breast. Korean J Intern Med 2006;21:199-201. [PubMed]
- Yoon G, Kim TJ, Sung CO, et al. Benign metastasizing leiomyoma with multiple lymph node metastasis: a case report. Cancer Res Treat 2011;43:131-3. [PubMed]
- Kang SA, Choi SI, Kim YA, et al. A case of benign metastasizing pulmonary leiomyoma. Tuberc Respir Dis 2005;58:614-8.
- Egberts JH, Schafmayer C, Bauerschlag DO, et al. Benign abdominal and pulmonary metastasizing leiomyoma of the uterus. Arch Gynecol Obstet 2006;274:319-22. [PubMed]
- Abramson S, Gilkeson RC, Goldstein JD, et al. Benign metastasizing leiomyoma: clinical, imaging, and pathologic correlation. AJR Am J Roentgenol 2001;176:1409-13. [PubMed]
- Patton KT, Cheng L, Papavero V, et al. Benign metastasizing leiomyoma: clonality, telomere length and clinicopathologic analysis. Mod Pathol 2006;19:130-40. [PubMed]
- Esteban JM, Allen WM, Schaerf RH. Benign metastasizing leiomyoma of the uterus: histologic and immunohistochemical characterization of primary and metastatic lesions. Arch Pathol Lab Med 1999;123:960-2. [PubMed]
- Jautzke G, Müller-Ruchholtz E, Thalmann U. Immunohistological detection of estrogen and progesterone receptors in multiple and well differentiated leiomyomatous lung tumors in women with uterine leiomyomas (so-called benign metastasizing leiomyomas). A report on 5 cases. Pathol Res Pract 1996;192:215-23. [PubMed]
- Nucci MR, Drapkin R, Dal Cin P, et al. Distinctive cytogenetic profile in benign metastasizing leiomyoma: pathogenetic implications. Am J Surg Pathol 2007;31:737-43. [PubMed]
- Tietze L, Günther K, Hörbe A, et al. Benign metastasizing leiomyoma: a cytogenetically balanced but clonal disease. Hum Pathol 2000;31:126-8. [PubMed]
- Nuovo GJ, Schmittgen TD. Benign metastasizing leiomyoma of the lung: clinicopathologic, immunohistochemical, and micro-RNA analyses. Diagn Mol Pathol 2008;17:145-50. [PubMed]
- Steiner PE. Metastasizing fibroleiomyoma of the uterus: Report of a case and review of the literature. Am J Pathol 1939;15:89-110.7.
- Robboy SJ, Bentley RC, Butnor K, et al. Pathology and pathophysiology of uterine smooth-muscle tumors. Environ Health Perspect 2000;108 Suppl 5:779-84. [PubMed]
- Canzonieri V, D’Amore ES, Bartoloni G, et al. Leiomyomatosis with vascular invasion. A unified pathogenesis regarding leiomyoma with vascular microinvasion, benign metastasizing leiomyoma and intravenous leiomyomatosis. Virchows Arch 1994;425:541-5. [PubMed]
- Koh DM, Burn PR, King DM. Benign metastasizing leiomyoma with intracaval leiomyomatosis. Br J Radiol 2000;73:435-7. [PubMed]
- Martin E. Leiomyomatous lung lesions: a proposed classification. AJR Am J Roentgenol 1983;141:269-72. [PubMed]
- Horstmann JP, Pietra GG, Harman JA, et al. Spontaneous regression of pulmonary leiomyomas during pregnancy. Cancer 1977;39:314-21. [PubMed]
- Fu Y, Li H, Tian B, et al. Pulmonary benign metastasizing leiomyoma: a case report and review of the literature. World J Surg Oncol 2012;10:268. [PubMed]
- Radzikowska E, Szczepulska-Wójcik E, Langfort R, et al. Benign pulmonary metastasizing leiomyoma uteri. Case report and review of literature. Pneumonol Alergol Pol 2012;80:560-4. [PubMed]
- Rivera JA, Christopoulos S, Small D, et al. Hormonal manipulation of benign metastasizing leiomyomas: report of two cases and review of the literature. J Clin Endocrinol Metab 2004;89:3183-8. [PubMed]
- Ahmad SZ, Anupama R, Vijaykumar DK. Benign metastasizing leiomyoma - case report and review of literature. Eur J Obstet Gynecol Reprod Biol 2011;159:240-1. [PubMed]
- Ni Y, Shi G, Wan H, et al. Pulmonary benign metastasizing leiomyoma: case report and review of the literature. Clin Exp Obstet Gynecol 2012;39:249-51. [PubMed]
- Goyle KK, Moore DF Jr, Garrett C, et al. Benign metastasizing leiomyomatosis: case report and review. Am J Clin Oncol 2003;26:473-6. [PubMed]
- Yamazaki K. CD10- and CD34-positive periglandular stromal cells in pulmonary benign metastasizing leiomyoma with metaplastic adenomyomatous glands: an ultrastructural and immunohistochemical study. Virchows Arch 2005;446:270-7. [PubMed]
- Yonezawa K, Yokoo N, Yamaguchi T. Effectiveness of an inferior vena caval filter as a preventive measure against pulmonary thromboembolism after abdominal surgery. Surg Today 1999;29:821-4. [PubMed]
- Abu-Rustum NR, Curtin JP, Burt M, et al. Regression of uterine low-grade smooth-muscle tumors metastatic to the lung after oophorectomy. Obstet Gynecol 1997;89:850-2. [PubMed]
- Mogi A, Hirato J, Kosaka T, et al. Benign metastasizing leiomyoma of the lung: report of a case. Gen Thorac Cardiovasc Surg 2013;61:719-22. [PubMed]
- Lewis EI, Chason RJ, DeCherney AH, et al. Novel hormone treatment of benign metastasizing leiomyoma: an analysis of five cases and literature review. Fertil Steril 2013;99:2017-24. [PubMed]