Published online Jun 7, 2018. doi: 10.3748/wjg.v24.i21.2247
Peer-review started: April 6, 2018
First decision: April 19, 2018
Revised: May 6, 2018
Accepted: May 18, 2018
Article in press: May 18, 2018
Published online: June 7, 2018
Every colorectal surgeon during his or her career is faced with anastomotic leakage (AL); one of the most dreaded complications following any type of gastrointestinal anastomosis due to increased risk of morbidity, mortality, overall impact on functional and oncologic outcome and drainage on hospital resources. In order to understand and give an overview of the AL risk factors in laparoscopic colorectal surgery, we carried out a careful review of the existing literature on this topic and found several different definitions of AL which leads us to believe that the lack of a consensual, standard definition can partly explain the considerable variations in reported rates of AL in clinical studies. Colorectal leak rates have been found to vary depending on the anatomic location of the anastomosis with reported incidence rates ranging from 0 to 20%, while the laparoscopic approach to colorectal resections has not yet been associated with a significant reduction in AL incidence. As well, numerous risk factors, though identified, lack unanimous recognition amongst researchers. For example, the majority of papers describe the risk factors for left-sided anastomosis, the principal risk being male sex and lower anastomosis, while little data exists defining AL risk factors in a right colectomy. Also, gut microbioma is gaining an emerging role as potential risk factor for leakage.
Core tip: In colorectal surgery, knowledge and prevention of possible complications are mandatory. Anastomotic leakage is a major issue in laparoscopic colorectal surgery and furthermore, its etiology is not fully understood. The aim of this review was to evaluate the current literature to identify patient-related and perioperative risk factors for leakage in patients undergoing colorectal resection by laparoscopy. Full awareness of risk factors is essential for identifying high-risk patients and properly select them for diverting stomas in order to mitigate potential severe clinical consequences of anastomotic leakage.
- Citation: Sciuto A, Merola G, De Palma GD, Sodo M, Pirozzi F, Bracale UM, Bracale U. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J Gastroenterol 2018; 24(21): 2247-2260
- URL: https://www.wjgnet.com/1007-9327/full/v24/i21/2247.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i21.2247
Every colorectal surgeon during his or her career is faced with anastomotic leakage (AL); one of the most dreaded complications following any type of gastrointestinal anastomosis due to increased risk of morbidity, mortality, overall impact on functional and oncologic outcome and drainage on hospital resources[1].
Several definitions of AL can be found in the literature and therefore lack of a standardized definition can partly explain the considerable variations in AL reported rates among clinical studies[1,2]. More generally, AL is grouped together with all conditions characterized by clinical or radiologic features of anastomotic dehiscence in accordance with the United Kingdom Surgical Infection Study Group[3-5]. In order to make a valid comparison of the different existing studies characterizing AL, in 2010 specific guidelines on defining AL following rectal surgery were published by the International Study Group of Rectal Cancer. According to these guidelines AL is defined as a defect of the intestinal wall at the anastomotic site (including suture and staple lines of neorectal reservoirs) leading to a communication between the intra- and extraluminal compartments[4].
The etiology of AL is considered multifactorial. Colorectal leak rates have been found to vary according to the anatomic location of the anastomosis, with distal colorectal, coloanal and ileoanal leak rates ranging from 1% to 20%, colocolonic leak rates from 0% to 2%, and ileocolonic leak rates from 0.02% to 4%[6-9]. After almost a century of investigation, a number of patient-related and perioperative factors, as well as technical considerations, have been implicated as risk factors for AL. In some instances conclusive recommendations are firmly justified whereas others are still open to debate[1,10]. Many authors have tried to compose nomograms in order to predict the risk of AL yet, despite the significance of such scores, they are not frequently used in clinical practice[11-13].
Surgical techniques and technologies as well as perioperative care have greatly evolved over the past several decades. The laparoscopic approach is now increasingly considered the standard of care in almost all colorectal diseases due to improved short-term postoperative results with no detrimental effects on oncological outcomes when compared to open surgery[14,15]. Laparoscopy is associated with providing a better view of the surgical field, less intraoperative blood loss, reduced tissue trauma and lower inflammatory response[16]. Despite these reported advantages the laparoscopic approach for colorectal resections has not been associated with a significant reduction in AL incidence until now. Most published studies and meta-analyses reported similar rates to open surgery[17,18]. Recently a retrospective analysis of 25097 patients undergoing colectomy for colon cancer revealed that, after adjusting for other factors, patients who had undergone open or converted procedures were nearly twice as likely to suffer from AL when compared to those subject to laparoscopy. This significant difference suggests that there may be true benefits to minimally invasive colon resection as it relates to AL[19].
The aim of this review was to evaluate the current literature in order to identify patient-related and perioperative risk factors for AL in patients undergoing colorectal resection by way of the laparoscopic approach.
A systematic review of literature was conducted according to the PRISMA statement[20]. A literature search was carried out in electronic databases (PubMed, MEDLINE, EMBASE) in order to retrieve all papers related to AL risk factors during laparoscopic colorectal surgery. The following search string was used: [(colorectal OR colon OR rectal OR colon surgery OR rectal surgery OR colorectal surgery) AND (anastomotic leak OR leakage OR fistula OR dehiscence) AND (risk factor OR risk) AND (laparoscopic OR laparoscopy)]. Two independent researchers analysed each article first by title and abstract, and subsequently by the full text and extracted the relevant data. In case of disagreement a third researcher was consulted. A manual search was conducted to identify further relevant studies. All papers not in the English language, reviews, meta-analyses and study-protocols were excluded. Both randomized and non-randomized studies were included in the review. The papers were divided into the following categories according to anastomosis location: (1) Right-sided anastomosis: all anastomoses involving the ileum and the colon such as in a right colectomy; (2) left-sided anastomosis: all anastomoses involving the left colon (colocolonic, colorectal and coloanal anastomoses) or the ileum (ileorectal and ileoanal); and (3) all types of resection: both right-sided and left-sided anastomoses.
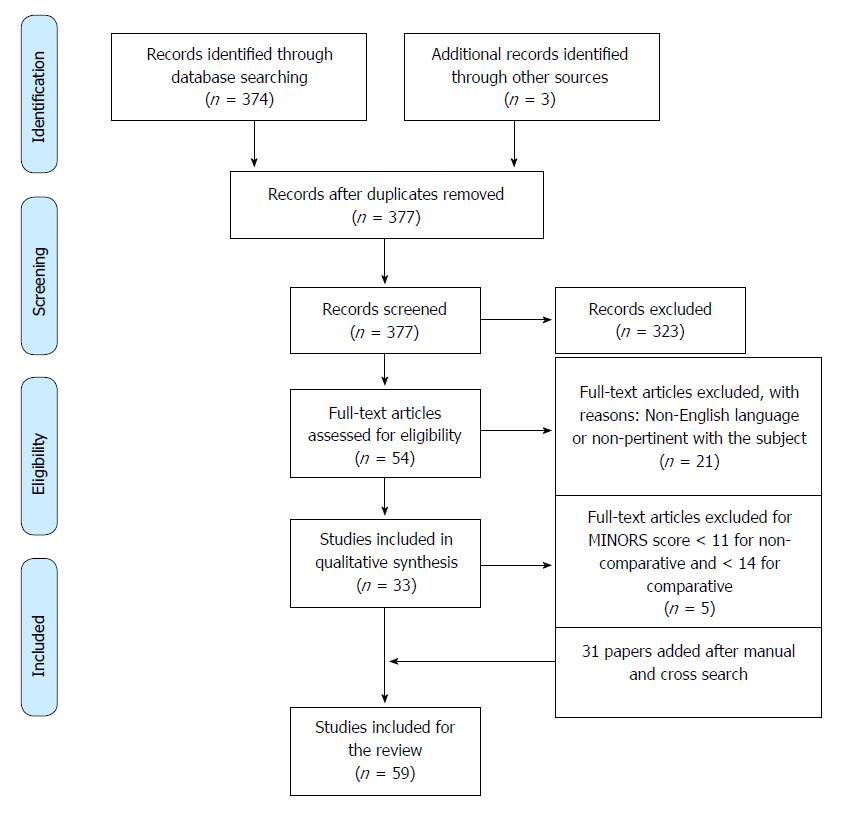
According to PRISMA guidelines, the selection flow diagram is reported in Figure 1.
The JADAD score was used to assess the quality of randomized controlled trials (RCTs) and papers with a score of ≥ 3 were included in the analysis. The methodological quality of non-randomized surgical studies was assessed with a MINORS score. A score ≥ 10 for non-comparative studies and ≥ 14 for comparative studies was fixed as a threshold for inclusion in the analysis[21,22].
After the literature review and quality assessment, one RCT and nine non-randomized papers were included in the analysis. Kwak et al[23] reported their retrospective series of 423 patients who had undergone laparoscopic colonic resection and anastomosis for appendix or right colon cancer. The overall leakage rate over the 8-year study period was 3.78% (16/423 patients). Among patient-related factors, habitual smoking was found to be significantly associated (P = 0.007) with AL with an odds ratio (OR) of 6.529 and it was suggested that vascular ischemia from nicotine-induced vasoconstriction and microthromboses, together with carbon monoxide-induced cellular hypoxia, inhibit anastomotic circulation in smokers[24]. Neoadjuvant chemotherapy correlated with AL (6.3% in the leakage group compared to the 0.5% in the non-leakage group, P = 0.007) however the sample size of only 3 patients was too small to be clinically relevant[23]. Among operative factors, longer operating time was found to be significantly associated with leakage (OR = 1.024, P < 0.001).
Laparoscopic right colectomy with intracorporeal anastomosis (IA) is reported to have some benefits in terms of enhanced postoperative recovery in comparison with laparoscopic-assisted right colectomy with extracorporeal anastomosis (EA)[25]. Both approaches appear to achieve similar results in terms of AL occurrence. Definitive conclusions are difficult to draw, however due to the nature of the published studies and the heterogeneity of surgical techniques used in fashioning the EA, including both manual, totally-stapled, and stapled-manual[26]. Vignali et al[26] published an interim analysis of the first RCT analyzing the role of intracorporeal stapled versus extracorporeal stapled anastomosis following laparoscopic right colectomy using a standardized approach. In their series of 60 patients (30 EA vs 30 IA) no significant difference was observed between the two groups with respect to AL (6.6% in the IA group versus 0% in the EA group, P = 0.39). In the largest multicenter study comparing IA and EA for 512 right-sided colorectal cancers, the incidence of leak or dehiscence was 4.19% (12 patients) in the IA group and 5.50% (12 patients) in the EA group (P = 0.53)[3]. Similarly, in a case-matched study, Vignali et al[27] compared the outcomes of IA (64 patients) versus EA (64 patients) in an obese population [body mass index (BMI) > 30 kg/m2]. Clinically evident anastomotic leaks occurred in 4.7% of the patients in the IA group vs 7.8% in the EA group (P = 0.71). Also, in a retrospective multicentric comparative study including 195 patients, multivariate analysis revealed a trend towards lower risk of clinical AL (requiring percutaneous or operative intervention) with IA that failed to reach statistical significance (adjusted OR = 0.29, P > 0.05)[28]. Other retrospective series found no significant differences in incidence of anastomotic leaks between the two techniques[29-32] . With regards to IA, a single-centre retrospective series of 162 patients found that double-layer closure of enterotomy was associated with a significantly lower incidence of AL compared to single-layer closure (1.2% in DL vs 7.8% in SL, P = 0.044) after mechanical ileocolic anastomosis[33].
Following a literature review and quality assessment, 5 RCTs and 34 non-randomized studies were included in the analysis (Table 1).
| Author | Year | No. of patients | Overall leak rate (n) | Risk factor identified |
| Ito et al[8] | 2008 | 180 | 5.0% (9) | TME |
| N° of staplers firing (≥ 3) | ||||
| Kim et al[36] | 2008 | 266 | 6.4% (17) | Male sex |
| Pugliese et al[66] | 2008 | 157 | 10.8% (17) | Conversion |
| Kim et al[47] | 2009 | 270 | 6.3% (17) | Tumor location in middle or lower rectum |
| Zhu et al[40] | 2010 | 132 | 9.1% (12) | Tumor size (diameter ≥ 3 cm) |
| Distance from the anal verge (≤ 6 cm) | ||||
| TNM stage | ||||
| Choi et al[45] | 2010 | 156 | 10.3% (16) | Anastomotic level ≤ 5 cm from the anal verge |
| Long operation time (≥ 270 min) | ||||
| Huh et al[46] | 2010 | 223 | 8.5% (19) | Extraperitoneal location of tumor |
| Operative time > 220 min | ||||
| Kayano et al[41] | 2011 | 250 | 10.0% (25) | Male sex |
| Multiple stapler firings (≥ 2) | ||||
| Akiyoshi et al[49] | 2011 | 363 | 3.6% (13) | Middle/low rectal cancer |
| Lack of pelvic drain | ||||
| Yamamoto et al[39] | 2012 | 111 | 5.4 (6) | BMI |
| Hinoi et al[68] | 2013 | 888 | 9.3% (83) | LCA ligation in LAR |
| Park et al[34] | 2013 | 1609 | 6.3% (101) | Male sex |
| Low anastomosis (< 7 cm) | ||||
| Preoperative chemoradiation | ||||
| Advanced tumor stage | ||||
| Perioperative bleeding (≥ 2 transfusions) | ||||
| Multiple firings of the linear stapler (> 3) | ||||
| Kawada et al[42] | 2014 | 154 | 12.3% (19) | Tumor size > 5 cm |
| Operative time > 300 min | ||||
| Intraoperative bleeding > 100 mL | ||||
| Stapler firings > 3 | ||||
| Precompression before stapler firing | ||||
| Majbar et al[65] | 2016 | 131 | 16.0% (21) | Conversion to open surgery |
| Silva-Velazco et al[38] | 2016 | 1059 | 9% (95) | BMI ≥ 35 kg/m2 |
| N° of staplers firing | ||||
| Longer operative time | ||||
| Van Praagh et al[74] | 2016 | 16 | 50% (8) | Low diversity of gut microbiota |
| High presence of Lachnospiraceae | ||||
| Hamabe et al[35] | 2017 | 296 | 8.1% (24) | Male sex |
| Distance from anal verge < 7 cm | ||||
| Neoadjuvant chemotherapy | ||||
| Lee et al[48] | 2017 | 128 | 0.78% (1) | Stapler firings > 2 |
| Distance from anal verge | ||||
| Tanaka et al[37] | 2017 | 395 | 8.4% (33) | Male sex |
| Absence of transanal tube | ||||
| Ito et al[44] | 2017 | 69 | 15.9% (11) | Absence of transanal tube |
| Post-operative diarrhea | ||||
| Shimura et al[43] | 2018 | 196 | 5.61% (11) | Post-operative hypoalbuminemia |
| Van Praagh et al[75] | 2018 | 123 | 23.6% (29) | Bacteroidaceae |
| Low diversity of gut microbiota | ||||
| High presence of Lachnospiraceae | ||||
| Anostomosis covered with C-Seal |
Male sex: AL was reported to be more common amongst men which may be reflective of the fact that technical difficulties can be intensified in male patients due to their narrow pelvises[34]. In a retrospective study of 296 patients who had undergone laparoscopic anterior resection (LAR), male gender was a significant risk factor with an OR of 18.0 at multivariate analysis[35]. Similarly, Kim et al[36] analyzed risk factors for AL in 312 LARs for both extraperitoneal and intraperitoneal disease location. Male gender was the only risk factor identified and leakage was 13.2 times higher in men than in women. Tanaka et al[37] ‘s prospective trial also found that men are at a higher risk for leakage (OR = 4.12). In a multicenter analysis of 1609 patients with rectal cancer, male gender was a significant risk factor amongst all patients [hazard ratio (HR) = 1.943] and particularly amongst patients without defunctioning stoma (HR = 3.468)[34].
BMI: Two papers have shown that BMI could also be a risk factor for AL. In a series of 1059 patients undergoing laparoscopic sigmoidectomy for diverticulitis, BMI ≥ 35 kg/m2 was independently associated (OR = 2.3) with AL and/or postoperative abscess both in an intent-to-treat analysis and amongst laparoscopically completed cases[38]. Yamamoto et al[39] found that BMI was independently predictive for developing AL (OR = 1.479).
Preoperative nutritional status: Malnutrition impairs anastomotic healing by affecting collagen synthesis or fibroblast proliferation. Impaired preoperative nutritious status defined as anemia or hypoproteinemia (hemoglobin ≤ 100 g/L or albumin ≤ 32 g/L) was found to be significant (P = 0.047) at a univariate analysis in a retrospective series of 132 patients undergoing LAR for cancer[40]. This finding was not confirmed at multivariate analysis (P = 0.253).
Neoadjuvant therapy: Park et al[34] reported that preoperative chemoradiation was a risk factor for leakage in their subgroup analysis of patients without defunctioning stoma (HR = 2.418), but not in their analysis of all patients after LAR for cancer. Hamabe et al[35] reported an association between AL and neoadjuvant chemotherapy with an OR of 3.5 at multivariate analysis.
Tumor size and stage: Tumor size may represent one of the risk factors for AL following LAR. This procedure involves surgery in an anatomically narrow space and as tumor size and/or stage increases, intrapelvic manipulation becomes restricted and rectal transection more challenging[41]. Moreover, patients with a tumor larger in size or more advanced in TNM staging usually suffer from a worsened systemic physical status[40]. In a series of 154 rectal cancer patients, tumor size ≥ 5 cm in diameter was associated with a 4-fold higher risk of leakage[42]. Zhu et al[40] found that tumors larger than 3 cm in diameter, as well as TNM stage, were independently associated with leakage.
Post-operative hypoalbuminemia: Post-operative nutritional status monitoring could be a good way to identify patients with high risk of post-operative AL. In a retrospective series of 200 patients undergoing laparoscopic curative surgery for colorectal cancer, the average serum albumin levels on POD1 and POD3 were significantly lower in the AL group compared to the non-leakage group (P < 0.0005)[43].
Post-operative diarrhea: Ito et al[44] reported an association between postoperative diarrhea and occurrence of AL, with an OR of 86.3. The authors speculated that early postoperative diarrhea increases endoluminal pressure at the anastomotic site. Furthermore, leaking of watery stool through the anastomosis may lead to the development of localized or generalized pelvic infection.
Level of anastomosis: The distance of the anastomosis from the anal verge is regarded as the most important predictive factor for leakage. Several studies have shown that the lower the anastomosis, the higher the risk of leakage[34,40,45-47]. Hamabe et al[35] reported that the leak rate was 3.4 times higher for tumors located less than 7 cm from the anal verge. An anastomotic level within 5 cm from the anal verge was a risk factor for leakage at both univariate (P < 0.001) and multivariate analysis (OR = 6.855; 95%CI: 1.271-36.964; P = 0.025) in a series of 156 patients undergoing LAR without diverting ileostomy[45]. In this study the AL rate was 10 times higher (20.6% vs 2.3%) when the anastomotic region was located within 5 cm of the anal verge. Accordingly, low levels of anastomosis accompanied with total mesorectal excision (TME) were independently associated with leakage[8]. In their series of 128 patients, Lee et al[48] reported that low distance from the anal verge could be a risk factor for leakage but that, due to their very low leak rate, they could not demonstrate it.
Number of linear stapler firings: A disadvantage in laparoscopic surgery is that rectal transection may be more difficult than in open surgery[41]. The narrow space in which to insert the stapler, inadequate traction and a suboptimal cutting angle may necessitate multiple applications of the linear stapler[34]. The concern about number and direction of stapler firings has been reported by many surgeons. In a series of 180 cancer patients, three or more stapler firings during rectal division significantly increased the risk of AL after the laparoscopic double stapling technique (OR = 4.6)[8]. Rectal division through the right-lower port required more stapler firings than division through the suprapubic port, especially in the TME group, and a smaller percentage of patients required three or more staples for vertical rectal division than for transverse division (15% vs 45%, P = 0.03). Park et al[34] also reported that a number of linear stapler firings > 3 was a risk factor for leakage (HR = 7.849). Choi et al[45] found that 16.7% of the cases in which 3 or more linear staplers were used had AL, whereas only 6.8% of the cases in which 2 or fewer linear staplers were used had leakage. Though there was no statistical significance to this difference (P = 0.068), the authors claimed that efforts to reduce the number of linear staplers to 2 or less seemed to be warranted. Kim et al[47] found that more than 2 stapler firings were associated with leakage at univariate analysis. The number of stapler firings increased significantly in men (P = 0.023), in patients with a tumor at a lower level (P = 0.034), and in those with longer operating times (P < 0.001). Several other authors reported an association between multiple linear stapler firings and AL incidence[38,41,42]. In Lee et al[48]’s series, this association could not be statistically proven due to the very low leak rate.
Diverting stoma: Although evidence regarding the clinical benefit of fecal diversion is conflicting, it is generally agreed that creation of a diverting stoma (DS) can reduce the clinically adverse effects of AL, including fecal peritonitis and septicemia, rather than preventing leakage. In a retrospective series of 69 patients undergoing LAR[44], no significant difference between DS group and no-DS group in terms of AL incidence (15.4% vs 16.3%) was noted. Although AL was observed in four patients in the DS group, none of them developed AL grade C. In contrast, 57.1% (4/7 cases) of the patients in the no-DS group developed AL grade C, but this difference did not reach statistical significance[44].
In the series from Park et al[34] (1609 patients) defunctioning stoma did not significantly reduce risk of AL (OR = 0.649, P = 0.154 at multivariate analysis). Similarly, in a series of 363 LARs, the incidence of AL was 4.8% in patients with covering stoma versus 3.3% in patients without stoma (P = 0.4718)[49] . Other studies reported similar findings[38,41,42].
In a series of 296 low LARs for cancer[35], AL was observed in 5.5% of patients with DS and in 8.7% of patients without DS (OR = 0.60, P = 0.4243 at univariate analysis). Based on the two risk factors (sex and anal verge distance) patients were stratified according to risk for AL occurrence. The incidence of AL was 8.1% in the overall population compared to 23% in high-risk patients (males with tumors less or equal than 7 cm from the anal verge). Within this group, DS creation significantly reduced the AL rate (P = 0.0363) as the rate of AL occurrence was 10.7% in patients for whom a DS was created compared to 33.3% in patients without a DS. The occurrence of AL in the low-risk group was not influenced by DS creation (P = 0.2443). Based on the findings of this study, DS may help prevent the occurrence of AL in a high-risk population.
Transanal TME: Transanal TME (TaTME) represents the latest advanced surgical access technique for pelvic dissection and anastomosis during rectal resection and is being implemented in clinical practice in order to overcome the technical drawbacks and limitations of standard laparoscopic TME[50] . For instance, the distal rectal transection does not involve multiple stapler firings and therefore eliminates this potential risk factor for leakage. Recently, Penna et al[50] analyzed 1594 TaTME cases with an anastomosis recorded on the international TaTME registry[51]. The overall anastomotic failure rate was 15.7%. This included early (within 30-d; 7.8%) and delayed (after 30 d; 2.0%) leak, pelvic abscess (4.7%), anastomotic fistula (0.8%), chronic sinus (0.9%), and anastomotic stricture in 3.6% of cases. Of 250 patients diagnosed with anastomotic failure, 219 had a defunctioning stoma created at the index operation. The reported early leak rate of 7.8% was higher than the previously published rate of 5.4% in the initial 720 registry cases[52]. The authors suggested that this value could be explained by an increased complexity of cases performed transanally, wider adoption of TaTME by surgeons at the start of their learning curve, or improved recording and reporting of adverse events on the registry. Nonetheless, the leak rate was comparable to previously reported incidences in colorectal surgery. Upon multivariate analysis, male sex, obesity, smoking, diabetes, larger tumors (> 25 mm maximum diameter), tumor height > 4 cm from anorectal junction on magnetic resonance imaging, and intraoperative blood loss of ≥ 500 mL were risk factors for early AL. These factors are similar to those identified in previous studies on laparoscopic rectal resections. Significantly more cases that did not have a defunctioning stoma developed early symptomatic AL compared with those that were defunctioned (12.4% vs 7.2%, OR = 0.547, P = 0.015). However, the presence of a defunctioning stoma did not appear to significantly influence incidence of anastomotic failure in this cohort. Anastomotic technique (manual versus stapled) was not identified as a risk factor for early AL, although the manual technique significantly increased the risk of late stricturing. A few published studies have compared laparoscopic and transanal TME with respect to AL rates. A RCT including 100 patients found a leak rate of 2% in the transanal group compared to 10% in the laparoscopic group, without a significant difference (P = 0.204)[53]. Other retrospective matched case-control trials did not find any statistically significant difference in terms of AL rates between the two approaches[54-57]. Results from the recently commenced RCTs comparing TaTME with laparoscopic TME may provide some robust data in the future[58,59].
Circular stapler: In animal models pre-compression before firing with a circular stapler was demonstrated to reduce intestinal wall thickness and acquire optimal anastomosis[42]. Only one published study reported that long pre-compression time before firing was associated with AL at a multivariate analysis (OR = 4.85)[42]. The diameter of the circular stapler was not found to be a risk factor for leakage in three studies[34,45,46].
Intraoperative endoscopy: The usual ways of assessing the integrity of colorectal anastomosis such as the air leak test, direct laparoscopic visualization and inspection of doughnuts may be suboptimal methods for predicting anastomotic complications. The use of intraoperative endoscopy (IOE) allows direct visualization and testing with the air leak test for anastomotic defect or bleeding, inadvertent bowel wall injury at the anastomotic site, adequacy of distal margins, vascularity of the anastomosis, and unsuspected distal lesions or stricture at the preoperative assessment[60]. Li et al[60]compared 107 patients who had undergone routine IOE to 137 patients who had undergone selective IOE during laparoscopic colorectal surgery. A 5.7-fold increase in anastomotic complications was observed in the selective IOE group although the difference was not statistically significant due to their small sample size. AL incidence was comparable between the two groups.
Indocyanine green fluorescence angiography: Intraoperative assessment of perfusion at the site of anastomosis with indocyanine green (ICG) has been increasingly considered a potential intraoperative tool that could be used to ensure adequate perfusion, possibly leading to a reduction in the AL rate. Most published studies focused on the change of surgical strategy (site of resection and/or anastomosis) due to the subjective recording of hypoperfusion after ICG fluorescence angiography (FA). However, its capacity to reduce AL incidence needs to be confirmed in large RCTs. Boni et al[61] compared 42 patients undergoing LAR with ICG angiography to a historical control group of 38 patients operated on without the use of angiography. No clinically relevant leaks were observed in the FA group, whereas two leaks were reported in the case-matched group. This difference is not likely to be statistically significant due to the limited number of patients analyzed. Jafari et al[62] published a prospective multicenter clinical trial including 139 patients who had undergone laparoscopic left-sided colectomy and anterior resection. The overall AL rate was 1.4%. FA changed surgical plans in 11 (7.9%) patients, with the majority of changes occurring at the time of transection of the proximal margin (7%). No AL was recorded amongst this subgroup of patients. In a prospective single-institution study of 68 patients undergoing laparoscopic resection for left-sided colorectal cancers, AL occurred in 16.7% of the poor perfusion group based on ICG fluorescence imaging, whereas none of the patients in the good perfusion group had AL. When further focusing on LAR, the AL rate was 10.7%. Leak occurred in 30% of the poor perfusion group, whereas no leak took place in the good perfusion group[63].
Fibrin glue: Fibrin glue application over the stapled anastomosis was not found to be significantly associated with leakage following laparoscopic rectal cancer surgery without stool diversion[46].
Operative time: Prolonged operations may reflect intraoperative difficulties especially in critical patients. Therefore operative time was investigated as a possible risk factor for AL. Silva Velazco et al[38] found an increasing OR of 1.03 for every 30 minutes of surgical duration. Several other authors have shown that prolonged operative time can be associated with leakage, with a reported threshold varying from 220 to 300 minutes[42,45,46].
Conversion: Conversion was found to be a controversial topic in the literature, with some authors reporting higher morbidity and mortality in converted patients, while others reporting outcomes comparable to laparoscopy. In a single-institution retrospective analysis of 1114 patients undergoing elective laparoscopic resection for non-metastatic colorectal cancer, the conversion rate was 10.9%. The most common reason for conversion was a locally advanced tumor followed by obesity and adhesions. Conversion was associated with significantly longer operative time and greater blood loss. No statistically significant differences in terms of an overall 30-day postoperative morbidity rate were observed between the converted and laparoscopic cases (16.4% vs 15.7%; P = 0.849) regardless of tumor location (colon vs rectum). In particular, no statistically significant differences were observed between the groups in terms of the AL rate (3.3% vs 4.9%; P = 0.416)[64].
In contrast, Majbar et al[65] in their retrospective study reported an association between conversion and AL at multivariate analysis (OR = 2.86). Similarly, in a series of 157 patients undergoing LAR for adenocarcinoma, Pugliese et al[66] reported a leak rate of 41% in converted patients compared to 8% in non-converted patients, with a 7.9-fold higher risk for developing a leak in the latter group.
Left colic artery ligation: The level of vascular ligation may affect blood supply to the anastomosis and subsequently anastomotic healing. Left colic artery (LCA) preservation results in increased blood supply for anastomosis after anterior resection, even in cases of the 5% of patients lacking a marginal artery in the left colic flexure resulting in ischemia on the proximal side of anastomosis[67]. The decision to perform a high or low tie of the inferior mesenteric artery during laparoscopic left-sided colorectal resections is controversial. In a multicenter retrospective study by 20 institutions in Japan, Hinoi et al[68] found that LCA preservation is a significant factor for low leakage rates after LAR for middle and low rectal cancers, regardless of tumor size, extent of lymph node metastasis, and extent of excision. In their series of 888 patients the overall incidence of anastomotic leak was 9.3%. LCA preservation was associated with a leak rate of 7.4% compared to 13.2% in the non-preservation group (P = 0.005 and < 0.001 by univariate and multivariate analysis, respectively) although this result might be biased due to the different surgical and pathological backgrounds between the two groups with more advanced cancer/stage in the LCA non-preservation group. Thus a subgroup analysis was performed on 411 patients undergoing en bloc radical lymph node excision associated with LCA ligation or preservation. The AL rate was 7.1% in the LCA preservation group compared to 14.5% in the LCA non-preservation group, the difference being statistically significant (P = 0.024 and 0.005, univariate and multivariate analysis, respectively). In contrast, the level of inferior mesenteric artery ligation was not found to be a risk factor for leakage in a series of 156 patients undergoing LAR without DS[45].
Pelvic drainage: Routine prophylactic drainage after colorectal anastomoses is debatable and the evidence to support its use is low[69]. A recent RCT analyzed 469 patients who underwent rectal resection with infraperitoneal anastomosis, of whom 93.6% were operated on by laparoscopy. There was no significant difference in terms of pelvic sepsis between drained and non-drained patients, either during hospital stay or at 30 days after surgery (16.1% vs 18.0%, P = 0.58). Early (< 5 d) versus late (> 5 d) pelvic drain removal did not affect significantly the risk of pelvic sepsis (11.6% vs 18.6%, P = 0.122)[70].Two retrospective studies found pelvic drainage associated with lower rates of AL after LAR, though without reaching statistical significance. Kawada et al[42] reported AL in 10.8% of drained patients versus 20.8% of non-drained patients (P = 0.18) in a series of 154 low LARs without DS. Similarly, in a series of 363 LARs, 2.6% of drained patients had clinical AL compared to 6.3% of non-drained patients (P = 0.11). Nonetheless lack of pelvic drain was found to be independently predictive (P = 0.0225, OR = 3.814) of leakage at a multivariate analysis[49]. Pelvic drain may prevent hematomas or seromas that constitute a fertile medium for bacteria and may promote infection which can involve the anastomosis thereby causing dehiscence. Moreover, pelvic drain may help control leaks if they do take place, leading to a less severe clinical course[71].
Trans-anal drainage: A trans-anal drainage tube was speculated by many authors to be a good way to prevent post-operative AL[37,44]. In a case series of 69 LARs, Ito et al[44] found that the use of trans-anal drainage is associated with lower incidence of post-operative AL. In particular, the authors explained that the presence of a trans-anal drain could prevent the unfavorable effect of post-operative diarrhea. Tanaka et al[37] also sustained that the absence of a trans-anal drainage tube after laparoscopic low anterior resection for stage 0/1 cancer is associated with a higher risk of post-operative AL with an OR of 3.11 at multivariate analysis. Contrarily, insertion of trans-anal drainage was reported as not correlating with AL by Hamabe et al[35], in high-risk patients as well.
Gut microbiota: Intestinal flora near the anastomotic site has been proposed to interact with intestinal tissue and likely affects intestinal healing[10]. Some experimental studies suggest that cues released by surgically injured tissues can lead to phenotype transformation of intraluminal microbes, turning them into pathogens. These may play a causative role in the development of AL by increased collagenase production and activation of host metalloproteinase-9[72]. Nonetheless, extensive clinical evidence on the impact of gut microbiota on postoperative anastomotic complications is lacking[73]. A pilot study compared the intestinal microbiota of 8 patients who had developed AL with 8 matched patients with healed circular stapled colorectal anastomoses without any clinical signs of AL[74]. The abundance of the Lachnospiraceae family was found to be significantly higher in patients who had developed AL when compared to patients who had not (P = 0.001), while microbial diversity levels were higher in the latter group (P = 0.037). Also, BMI was positively associated with the abundance of the Lachnospiraceae family (P = 0.022). The same study group further investigated the role of gut microbiota in the development of AL in a series of 123 ‘‘donuts’’ of patients where a stapled colorectal anastomosis was made[75]. In 63 patients this anastomosis was covered with a C-seal; a bioresorbable sheath stapled to the anastomosis. In the group of non-C-seal samples a high abundance of Lachnospiraceae and Bacteroidaceae and lower microbial diversity were confirmed to be strongly associated with AL. A bacterial composition that consisted of 60% or more of these two families seemed to be predictive for AL. On the contrary, other species such as Prevotella copri and the Streptococcus genus were both negatively associated with AL. The authors speculated that a disturbed microbial composition which is more easily associated with low microbial diversity[10] due to preoperative or surgical processes, may affect the metabolic balance and lack colonization resistance to pathogenic bacteria that could play a role in the development of AL. In C-seal patients where AL rates were slightly higher, it seemed that any potential protective benefits or harmful consequences of the gut microbiota composition were negated, as progression to AL was independent of the dominant bacterial composition before surgery. These observations suggested that the C-seal influences the microbial composition after introduction and that this may ultimately impair anastomotic healing.
Perioperative events: Bleeding during surgery may predispose to leakage due to hemodynamic alterations at the anastomotic site. Kawada et al[42] found that intraoperative bleeding at more than 100 mL was associated with significantly increased incidence of leakage (P = 0.037). Perioperative bleeding requiring 2 or more units was reported to be a risk factor for leakage in patients undergoing LAR for cancer (HR = 8.462) including those without defunctioning stoma (HR = 10.705)[34]. Also, unexpected events related to anastomosis during surgery such as instrument failure, ischemia of the proximal colon, tumor perforation and additional surgery caused by anastomotic bleeding have been significantly associated with leakage[45].
Surgeon’s experience and hospital size: Two important factors that may impact the risk of AL after laparoscopic colorectal surgery are the experience of the surgeon performing the procedure and hospital volume. Two published papers report on the risk of AL as related to the experience of the surgeon and only one related to hospital size[76-78]. The individual surgeon performing the procedure, as well as hospital volume, were found to be risk factors for AL although these studies were excluded from the review after quality assessment.
Kayano et al[41] analyzed the AL rate of LAR during the learning curve period in a series of 250 cases that were evaluated in five groups of 50 patients each. The postoperative complication rate decreased significantly by group 5 (201-250 cases) and it was noted that AL decreased with an increase in cases although no significant difference was observed over the course of the learning curve period. Park et al[34] found no correlation between the incidence of AL and both hospital caseload and surgeon’s TME experience.
After the literature review and quality assessment, three RCTs and seven non-randomized studies were included in the analysis (Table 2).
| Author | Year | No. of patients | Overall leak rate (n) | Risk factor identified |
| Kockerling et al[82] | 1999 | 894 | 4.2% (38) | Rectal resection |
| Malignant disease | ||||
| Anastomotic level < 10 cm from the anal verge | ||||
| Senagore et al[80] | 2003 | 260 | 2.7% (7) | BMI ≥ 30 kg/m2 |
| Kirchhoff et al[83] | 2008 | 1316 | 27.7% (59) | BMI ≥ 30 kg/m2 |
| Male gender | ||||
| Malignant neoplasia | ||||
| Akiyoshi et al[79] | 2011 | 1194 | 1.0% (12) | BMI ≥ 30 kg/m2 |
| Rectal tumor location | ||||
| Ris et al[90] | 2018 | 504 | 2.4% (12) | No use of indocyanine green |
BMI: In a cohort of 1194 patients who had undergone laparoscopic resection for colorectal cancer, the rate of AL was significantly higher in the obese II Group (BMI > 30 kg/m2) than in the nonobese (< 24.9 kg/m2) and obese I (BMI 25 to 29.9 kg/m2) groups (8% vs 1% and 0.4%; P = 0.0004 and 0.0002, respectively). BMI > 30 kg/m2 was found to be independently predictive of the development of leakage (OR = 10.27)[79]. Similarly, in a series of 260 laparoscopic colectomies, the AL rate was significantly higher amongst obese (5.1%) versus non-obese (1.2%) patients[80]. On the contrary, a retrospective study on 213 patients undergoing laparoscopy colorectal surgery for inflammatory bowel disease failed to demonstrate any difference in AL rates between normal-weight patients and overweight or obese patients[81].
Tumor location: Akiyoshi et al[79] reported that tumor location in the rectum, rather in the colon, was found to be independently predictive of the development of AL (OR = 18.20) upon multivariate analysis. At univariate analysis, the type of operative procedure (LAR/intersphincteric resection versus others) was associated with leakage (P = 0.0004) in addition to tumor location. This finding was confirmed by a prospective multicenter study which reported on 1134 patients of whom 894 had an anastomosis[82]. In this series the leak rate was highest after LAR (12.7%) followed by left hemicolectomy (7.1%), right hemicolectomy (4%), sigmoidectomy (2.9%), and rectopexy with resection (1.25%; P = 0.0001). Surgery for benign disease was associated with a lower rate of AL (2.6%) than surgery for malignant disease (6.7%). Cancer was significantly associated with AL in a series of 1316 elective laparoscopic colorectal procedures as well[83].
Preoperative Infliximab therapy: In a retrospective series of patients undergoing elective laparoscopic resection for inflammatory bowel disease, 142 had preoperative therapy within 12 wk before surgery and were compared to 376 who had not received Infliximab. The rate of anastomotic leaks (2.1% vs 1.3%, P = 0.81) was similar. Subgroup analysis confirmed similar rates of leakage regardless of whether patients had ulcerative colitis or Crohn’s disease. According to this study, Infliximab treatment in patients refractory to conventional pharmacological therapy did not seem to affect short-term outcomes in those patients eventually submitted to surgical treatment[84].
Oral antibiotics: Recent studies[85,86] suggest that use of oral antibiotics in preoperative bowel preparation could lower infectious complications and also incidence of AL after colorectal surgery. This finding further supports a role of the gut microbiota in anastomotic integrity[67]. However data on the impact of this measure in patients specifically undergoing minimally invasive colorectal surgery are still limited[86]. In a retrospective ACS-NSQIP database analysis, in which 5291 (62.5%) patients underwent minimally invasive surgery, oral antibiotic preparation was associated with lower rates of surgical site infection (SSI) and AL for both minimally invasive and open cohorts[87]. A recent RCT by Hata et al[88] revealed that patients undergoing laparoscopic colorectal procedures for cancer had a lower incidence of overall SSIs (7.3% vs 12.8%, OR = 0.536, P = 0.028) when receiving oral antibiotic prophylaxis in addition to mechanical bowel preparation. However, incidence of organ/space infection was comparable to that of patients receiving mechanical bowel preparation and IV prophylaxis where 6/290 (2.1%) leaks took place in the IV group compared to 5/289 (1.7%) in the oral-IV group. In another single-center RCT including 515 colorectal cancer patients undergoing elective laparoscopic resection, IV perioperative antimicrobial prophylaxis alone was not inferior to combined pre-operative oral and IV perioperative prophylaxis with regards to SSI. AL was observed in 2.5% of the IV-only group and in 1.2% of the oral-IV group (OR = 2.01, P = 0.504). The authors speculated that the study was evidently underpowered to provide any conclusions regarding the contribution of oral microbial prophylaxis in reducing AL[89].
Indocyanine green fluorescence angiography: Ris et al[90] recently conducted a prospective phase II study of 504 patients undergoing elective bowel resection of which 85.3% were operated on by laparoscopy. The overall leak rate for colorectal operations not involving ICG fluorescence was 5.8%, compared with 2.6% with the use of ICG imaging (P = 0.009). Statistical significance was confirmed for left-sided resections (6.9% vs 2.6%, P = 0.005) and for low anterior resections alone (10.7% vs 3%), but not for right-sided operations (2.6% vs 2.8%, P = 0.928).
Some limitations of this study have to be addressed. The major limitation lies in the retrospective nature and consequent lack of randomization of the included studies, that may lead to patient and surgeon selection bias. Second, different definitions of AL were used across the studies, which is a general problem in the literature dealing with this postoperative complication. Moreover, some series are heterogeneous in terms of type of patients, study era, surgical technique, and perioperative practice. The variable presence of DS across studies dealing with rectal resections should also be considered. Finally, some studies have relatively small sample size.
Anastomotic leakage remains a major issue in laparoscopic colorectal surgery. Current evidence about the risk factors for leaking mainly comes from non-randomized retrospective studies, most of which deal with rectal resections. In such studies, the presence of a diverting stoma should be taken into account when analysing the association between leakage and predictive factors. Several clinical variables and surgical issues have been extensively investigated, although some of them remain controversial, and it remains difficult to accurately predict the development of leakage. This suggests that the etiology of this fearsome complication is not fully understood and dictates the need for further investigations. Full awareness of risk factors is essential for identifying high-risk patients and properly select them for diverting stomas in order to mitigate the severe clinical consequences of anastomotic leakage.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: García-Flórez LJ, Komatsu S, Lieske B S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Chadi SA, Fingerhut A, Berho M, DeMeester SR, Fleshman JW, Hyman NH, Margolin DA, Martz JE, McLemore EC, Molena D. Emerging Trends in the Etiology, Prevention, and Treatment of Gastrointestinal Anastomotic Leakage. J Gastrointest Surg. 2016;20:2035-2051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 120] [Article Influence: 15.0] [Reference Citation Analysis (2)] |
| 2. | Carlomagno N, Santangelo ML, Amato B, Calogero A, Saracco M, Cremone C, Miranda A, Dodaro C, Renda A. Total colectomy for cancer: analysis of factors linked to patients’ age. Int J Surg. 2014;12 Suppl 2:S135-S139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (2)] |
| 3. | Milone M, Elmore U, Di Salvo E, Delrio P, Bucci L, Ferulano GP, Napolitano C, Angiolini MR, Bracale U, Clemente M. Intracorporeal versus extracorporeal anastomosis. Results from a multicentre comparative study on 512 right-sided colorectal cancers. Surg Endosc. 2015;29:2314-2320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 103] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 4. | Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 732] [Cited by in F6Publishing: 787] [Article Influence: 56.2] [Reference Citation Analysis (3)] |
| 5. | Peel AL, Taylor EW. Proposed definitions for the audit of postoperative infection: a discussion paper. Surgical Infection Study Group. Ann R Coll Surg Engl. 1991;73:385-388. [PubMed] [Cited in This Article: ] |
| 6. | Phitayakorn R, Delaney CP, Reynolds HL, Champagne BJ, Heriot AG, Neary P, Senagore AJ; International Anastomotic Leak Study Group. Standardized algorithms for management of anastomotic leaks and related abdominal and pelvic abscesses after colorectal surgery. World J Surg. 2008;32:1147-1156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA. Anastomotic leaks after intestinal anastomosis: it’s later than you think. Ann Surg. 2007;245:254-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 420] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 8. | Ito M, Sugito M, Kobayashi A, Nishizawa Y, Tsunoda Y, Saito N. Relationship between multiple numbers of stapler firings during rectal division and anastomotic leakage after laparoscopic rectal resection. Int J Colorectal Dis. 2008;23:703-707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 9. | Crafa F, Smolarek S, Missori G, Shalaby M, Quaresima S, Noviello A, Cassini D, Ascenzi P, Franceschilli L, Delrio P. Transanal Inspection and Management of Low Colorectal Anastomosis Performed With a New Technique: the TICRANT Study. Surg Innov. 2017;24:483-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Shogan BD, Carlisle EM, Alverdy JC, Umanskiy K. Do we really know why colorectal anastomoses leak? J Gastrointest Surg. 2013;17:1698-1707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 11. | Yao HH, Shao F, Huang Q, Wu Y, Qiang Zhu Z, Liang W. Nomogram to predict anastomotic leakage after laparoscopic anterior resection with intracorporeal rectal transection and double-stapling technique anastomosis for rectal cancer. Hepatogastroenterology. 2014;61:1257-1261. [PubMed] [Cited in This Article: ] |
| 12. | Kim CH, Lee SY, Kim HR, Kim YJ. Nomogram Prediction of Anastomotic Leakage and Determination of an Effective Surgical Strategy for Reducing Anastomotic Leakage after Laparoscopic Rectal Cancer Surgery. Gastroenterol Res Pract. 2017;2017:4510561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Rencuzogullari A, Benlice C, Valente M, Abbas MA, Remzi FH, Gorgun E. Predictors of Anastomotic Leak in Elderly Patients After Colectomy: Nomogram-Based Assessment From the American College of Surgeons National Surgical Quality Program Procedure-Targeted Cohort. Dis Colon Rectum. 2017;60:527-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Bracale U, Sodo M, Merola G, Di Salvo E. Reply to Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. ESMO Open. 2016;1:e000110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Bracale U, Melillo P, Lazzara F, Andreuccetti J, Stabilini C, Corcione F, Pignata G. Single-access laparoscopic rectal resection versus the multiport technique: a retrospective study with cost analysis. Surg Innov. 2015;22:46-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Bissolati M, Orsenigo E, Staudacher C. Minimally invasive approach to colorectal cancer: an evidence-based analysis. Updates Surg. 2016;68:37-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Arezzo A, Passera R, Scozzari G, Verra M, Morino M. Laparoscopy for rectal cancer reduces short-term mortality and morbidity: results of a systematic review and meta-analysis. Surg Endosc. 2013;27:1485-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Abraham NS, Young JM, Solomon MJ. Meta-analysis of short-term outcomes after laparoscopic resection for colorectal cancer. Br J Surg. 2004;91:1111-1124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 468] [Cited by in F6Publishing: 504] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 19. | Mungo B, Papageorge CM, Stem M, Molena D, Lidor AO. The Impact of Operative Approach on Postoperative Complications Following Colectomy for Colon Caner. World J Surg. 2017;41:2143-2152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7677] [Cited by in F6Publishing: 8440] [Article Influence: 562.7] [Reference Citation Analysis (0)] |
| 21. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [PubMed] [Cited in This Article: ] |
| 22. | Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [PubMed] [Cited in This Article: ] |
| 23. | Kwak HD, Kim SH, Kang DW, Baek SJ, Kwak JM, Kim J. Risk Factors and Oncologic Outcomes of Anastomosis Leakage After Laparoscopic Right Colectomy. Surg Laparosc Endosc Percutan Tech. 2017;27:440-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Sørensen LT, Jørgensen T, Kirkeby LT, Skovdal J, Vennits B, Wille-Jørgensen P. Smoking and alcohol abuse are major risk factors for anastomotic leakage in colorectal surgery. Br J Surg. 1999;86:927-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 247] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 25. | Bracale U, Merola G, Cabras F, Andreuccetti J, Corcione F, Pignata G. The Use of Barbed Suture for Intracorporeal Mechanical Anastomosis During a Totally Laparoscopic Right Colectomy: Is It Safe? A Retrospective Nonrandomized Comparative Multicenter Study. Surg Innov. 2018;25:267-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Vignali A, Bissolati M, De Nardi P, Di Palo S, Staudacher C. Extracorporeal vs. Intracorporeal Ileocolic Stapled Anastomoses in Laparoscopic Right Colectomy: An Interim Analysis of a Randomized Clinical Trial. J Laparoendosc Adv Surg Tech A. 2016;26:343-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Vignali A, Elmore U, Lemma M, Guarnieri G, Radaelli G, Rosati R. Intracorporeal versus Extracorporeal Anastomoses Following Laparoscopic Right Colectomy in Obese Patients: A Case-Matched Study. Dig Surg. 2018;35:236-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Hanna MH, Hwang GS, Phelan MJ, Bui TL, Carmichael JC, Mills SD, Stamos MJ, Pigazzi A. Laparoscopic right hemicolectomy: short- and long-term outcomes of intracorporeal versus extracorporeal anastomosis. Surg Endosc. 2016;30:3933-3942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Biondi A, Santocchi P, Pennestrì F, Santullo F, D’Ugo D, Persiani R. Totally laparoscopic right colectomy versus laparoscopically assisted right colectomy: a propensity score analysis. Surg Endosc. 2017;31:5275-5282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Vergis AS, Steigerwald SN, Bhojani FD, Sullivan PA, Hardy KM. Laparoscopic right hemicolectomy with intracorporeal versus extracorporeal anastamosis: a comparison of short-term outcomes. Can J Surg. 2015;58:63-68. [PubMed] [Cited in This Article: ] |
| 31. | Magistro C, Lernia SD, Ferrari G, Zullino A, Mazzola M, De Martini P, De Carli S, Forgione A, Bertoglio CL, Pugliese R. Totally laparoscopic versus laparoscopic-assisted right colectomy for colon cancer: is there any advantage in short-term outcomes? A prospective comparative assessment in our center. Surg Endosc. 2013;27:2613-2618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Shapiro R, Keler U, Segev L, Sarna S, Hatib K, Hazzan D. Laparoscopic right hemicolectomy with intracorporeal anastomosis: short- and long-term benefits in comparison with extracorporeal anastomosis. Surg Endosc. 2016;30:3823-3829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 33. | Reggio S, Sciuto A, Cuccurullo D, Pirozzi F, Esposito F, Cusano D, Corcione F. Single-layer versus double-layer closure of the enterotomy in laparoscopic right hemicolectomy with intracorporeal anastomosis: a single-center study. Tech Coloproctol. 2015;19:745-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY, Kang SB, Kim JY, Lee KY, Kim BC. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg. 2013;257:665-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 293] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 35. | Hamabe A, Ito M, Nishigori H, Nishizawa Y, Sasaki T. Preventive effect of diverting stoma on anastomotic leakage after laparoscopic low anterior resection with double stapling technique reconstruction applied based on risk stratification. Asian J Endosc Surg. 2017; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Kim SH, Park IJ, Joh YG, Hahn KY. Laparoscopic resection of rectal cancer: a comparison of surgical and oncologic outcomes between extraperitoneal and intraperitoneal disease locations. Dis Colon Rectum. 2008;51:844-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Tanaka K, Okuda J, Yamamoto S, Ito M, Sakamoto K, Kokuba Y, Yoshimura K, Watanabe M. Risk factors for anastomotic leakage after laparoscopic surgery with the double stapling technique for stage 0/I rectal carcinoma: a subgroup analysis of a multicenter, single-arm phase II trial. Surg Today. 2017;47:1215-1222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Silva-Velazco J, Stocchi L, Costedio M, Gorgun E, Kessler H, Remzi FH. Is there anything we can modify among factors associated with morbidity following elective laparoscopic sigmoidectomy for diverticulitis? Surg Endosc. 2016;30:3541-3551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Yamamoto S, Fujita S, Akasu T, Inada R, Moriya Y, Yamamoto S. Risk factors for anastomotic leakage after laparoscopic surgery for rectal cancer using a stapling technique. Surg Laparosc Endosc Percutan Tech. 2012;22:239-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Zhu QL, Feng B, Lu AG, Wang ML, Hu WG, Li JW, Mao ZH, Zheng MH. Laparoscopic low anterior resection for rectal carcinoma: complications and management in 132 consecutive patients. World J Gastroenterol. 2010;16:4605-4610. [PubMed] [Cited in This Article: ] |
| 41. | Kayano H, Okuda J, Tanaka K, Kondo K, Tanigawa N. Evaluation of the learning curve in laparoscopic low anterior resection for rectal cancer. Surg Endosc. 2011;25:2972-2979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 98] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 42. | Kawada K, Hasegawa S, Hida K, Hirai K, Okoshi K, Nomura A, Kawamura J, Nagayama S, Sakai Y. Risk factors for anastomotic leakage after laparoscopic low anterior resection with DST anastomosis. Surg Endosc. 2014;28:2988-2995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 43. | Shimura T, Toiyama Y, Hiro J, Imaoka H, Fujikawa H, Kobayashi M, Ohi M, Inoue Y, Mohri Y, Kusunoki M. Monitoring perioperative serum albumin can identify anastomotic leakage in colorectal cancer patients with curative intent. Asian J Surg. 2018;41:30-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Ito T, Obama K, Sato T, Matsuo K, Inoue H, Kubota K, Tamaki N, Kami K, Yoshimura N, Shono T. Usefulness of transanal tube placement for prevention of anastomotic leakage following laparoscopic low anterior resection. Asian J Endosc Surg. 2017;10:17-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Choi DH, Hwang JK, Ko YT, Jang HJ, Shin HK, Lee YC, Lim CH, Jeong SK, Yang HK. Risk factors for anastomotic leakage after laparoscopic rectal resection. J Korean Soc Coloproctol. 2010;26:265-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Huh JW, Kim HR, Kim YJ. Anastomotic leakage after laparoscopic resection of rectal cancer: the impact of fibrin glue. Am J Surg. 2010;199:435-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 47. | Kim JS, Cho SY, Min BS, Kim NK. Risk factors for anastomotic leakage after laparoscopic intracorporeal colorectal anastomosis with a double stapling technique. J Am Coll Surg. 2009;209:694-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 48. | Lee S, Ahn B, Lee S. The Relationship Between the Number of Intersections of Staple Lines and Anastomotic Leakage After the Use of a Double Stapling Technique in Laparoscopic Colorectal Surgery. Surg Laparosc Endosc Percutan Tech. 2017;27:273-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Akiyoshi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Y, Konishi T, Kuroyanagi H, Yamaguchi T. Incidence of and risk factors for anastomotic leakage after laparoscopic anterior resection with intracorporeal rectal transection and double-stapling technique anastomosis for rectal cancer. Am J Surg. 2011;202:259-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 50. | Penna M, Hompes R, Arnold S, Wynn G, Austin R, Warusavitarne J, Moran B, Hanna GB, Mortensen NJ, Tekkis PP; International TaTME Registry Collaborative. Incidence and Risk Factors for Anastomotic Failure in 1594 Patients Treated by Transanal Total Mesorectal Excision: Results From the International TaTME Registry. Ann Surg. 2018; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 208] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 51. | Hompes R, Arnold S, Warusavitarne J. Towards the safe introduction of transanal total mesorectal excision: the role of a clinical registry. Colorectal Dis. 2014;16:498-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Penna M, Hompes R, Arnold S, Wynn G, Austin R, Warusavitarne J, Moran B, Hanna GB, Mortensen NJ, Tekkis PP; TaTME Registry Collaborative. Transanal Total Mesorectal Excision: International Registry Results of the First 720 Cases. Ann Surg. 2017;266:111-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 304] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 53. | Denost Q, Adam JP, Rullier A, Buscail E, Laurent C, Rullier E. Perineal transanal approach: a new standard for laparoscopic sphincter-saving resection in low rectal cancer, a randomized trial. Ann Surg. 2014;260:993-999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 54. | de’Angelis N, Portigliotti L, Azoulay D, Brunetti F. Transanal total mesorectal excision for rectal cancer: a single center experience and systematic review of the literature. Langenbecks Arch Surg. 2015;400:945-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 55. | Kanso F, Maggiori L, Debove C, Chau A, Ferron M, Panis Y. Perineal or Abdominal Approach First During Intersphincteric Resection for Low Rectal Cancer: Which Is the Best Strategy? Dis Colon Rectum. 2015;58:637-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 56. | Fernández-Hevia M, Delgado S, Castells A, Tasende M, Momblan D, Díaz del Gobbo G, DeLacy B, Balust J, Lacy AM. Transanal total mesorectal excision in rectal cancer: short-term outcomes in comparison with laparoscopic surgery. Ann Surg. 2015;261:221-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 233] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 57. | Perdawood SK, Al Khefagie GA. Transanal vs laparoscopic total mesorectal excision for rectal cancer: initial experience from Denmark. Colorectal Dis. 2016;18:51-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 58. | Deijen CL, Velthuis S, Tsai A, Mavroveli S, de Lange-de Klerk ES, Sietses C, Tuynman JB, Lacy AM, Hanna GB, Bonjer HJ. COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc. 2016;30:3210-3215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 59. | Lelong B, de Chaisemartin C, Meillat H, Cournier S, Boher JM, Genre D, Karoui M, Tuech JJ, Delpero JR; French Research Group of Rectal Cancer Surgery (GRECCAR). A multicentre randomised controlled trial to evaluate the efficacy, morbidity and functional outcome of endoscopic transanal proctectomy versus laparoscopic proctectomy for low-lying rectal cancer (ETAP-GRECCAR 11 TRIAL): rationale and design. BMC Cancer. 2017;17:253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 60. | Li VK, Wexner SD, Pulido N, Wang H, Jin HY, Weiss EG, Nogeuras JJ, Sands DR. Use of routine intraoperative endoscopy in elective laparoscopic colorectal surgery: can it further avoid anastomotic failure? Surg Endosc. 2009;23:2459-2465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Boni L, Fingerhut A, Marzorati A, Rausei S, Dionigi G, Cassinotti E. Indocyanine green fluorescence angiography during laparoscopic low anterior resection: results of a case-matched study. Surg Endosc. 2017;31:1836-1840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 62. | Jafari MD, Wexner SD, Martz JE, McLemore EC, Margolin DA, Sherwinter DA, Lee SW, Senagore AJ, Phelan MJ, Stamos MJ. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg. 2015;220:82-92.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 345] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 63. | Kawada K, Hasegawa S, Wada T, Takahashi R, Hisamori S, Hida K, Sakai Y. Evaluation of intestinal perfusion by ICG fluorescence imaging in laparoscopic colorectal surgery with DST anastomosis. Surg Endosc. 2017;31:1061-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 64. | Allaix ME, Degiuli M, Arezzo A, Arolfo S, Morino M. Does conversion affect short-term and oncologic outcomes after laparoscopy for colorectal cancer? Surg Endosc. 2013;27:4596-4607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 65. | Majbar AM, Abid M, Alaoui M, Sabbah F, Raiss M, Ahallat M, Hrora A. Impact of Conversion to Open Surgery on Early Postoperative Morbidity After Laparoscopic Resection for Rectal Adenocarcinoma: A Retrospective Study. J Laparoendosc Adv Surg Tech A. 2016;26:697-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Pugliese R, Di Lernia S, Sansonna F, Scandroglio I, Maggioni D, Ferrari GC, Costanzi A, Magistro C, De Carli S. Results of laparoscopic anterior resection for rectal adenocarcinoma: retrospective analysis of 157 cases. Am J Surg. 2008;195:233-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Kachlik D, Baca V. Macroscopic and microscopic intermesenteric communications. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:121-124. [PubMed] [Cited in This Article: ] |
| 68. | Hinoi T, Okajima M, Shimomura M, Egi H, Ohdan H, Konishi F, Sugihara K, Watanabe M. Effect of left colonic artery preservation on anastomotic leakage in laparoscopic anterior resection for middle and low rectal cancer. World J Surg. 2013;37:2935-2943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 69. | Emile SH, Abd El-Hamed TM. Routine Drainage of Colorectal Anastomoses: An Evidence-Based Review of the Current Literature. Gastroenterol Res Pract. 2017;2017:6253898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Denost Q, Rouanet P, Faucheron JL, Panis Y, Meunier B, Cotte E, Meurette G, Kirzin S, Sabbagh C, Loriau J, Benoist S, Mariette C, Sielezneff I, Lelong B, Mauvais F, Romain B, Barussaud ML, Germain C, Picat MQ, Rullier E, Laurent C; French Research Group of Rectal Cancer Surgery (GRECCAR). To Drain or Not to Drain Infraperitoneal Anastomosis After Rectal Excision for Cancer: The GRECCAR 5 Randomized Trial. Ann Surg. 2017;265:474-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 71. | Qu H, Liu Y, Bi DS. Clinical risk factors for anastomotic leakage after laparoscopic anterior resection for rectal cancer: a systematic review and meta-analysis. Surg Endosc. 2015;29:3608-3617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 72. | Shogan BD, Belogortseva N, Luong PM, Zaborin A, Lax S, Bethel C, Ward M, Muldoon JP, Singer M, An G. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med. 2015;7:286ra68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 247] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 73. | Russ AJ, Casillas MA. Gut Microbiota and Colorectal Surgery: Impact on Postoperative Complications. Clin Colon Rectal Surg. 2016;29:253-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | van Praagh JB, de Goffau MC, Bakker IS, Harmsen HJ, Olinga P, Havenga K. Intestinal microbiota and anastomotic leakage of stapled colorectal anastomoses: a pilot study. Surg Endosc. 2016;30:2259-2265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 75. | van Praagh JB, de Goffau MC, Bakker IS, van Goor H, Harmsen HJM, Olinga P, Havenga K. Mucus Microbiome of Anastomotic Tissue During Surgery Has Predictive Value for Colorectal Anastomotic Leakage. Ann Surg. 2018; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 76. | Marinello FG, Baguena G, Lucas E, Frasson M, Hervás D, Flor-Lorente B, Esclapez P, Espí A, García-Granero E. Anastomotic leakage after colon cancer resection: does the individual surgeon matter? Colorectal Dis. 2016;18:562-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 77. | García-Granero E, Navarro F, Cerdán Santacruz C, Frasson M, García-Granero A, Marinello F, Flor-Lorente B, Espí A. Individual surgeon is an independent risk factor for leak after double-stapled colorectal anastomosis: An institutional analysis of 800 patients. Surgery. 2017;162:1006-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 78. | Nikolian VC, Kamdar NS, Regenbogen SE, Morris AM, Byrn JC, Suwanabol PA, Campbell DA Jr, Hendren S. Anastomotic leak after colorectal resection: A population-based study of risk factors and hospital variation. Surgery. 2017;161:1619-1627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 82] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Akiyoshi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Y, Konishi T, Kuroyanagi H, Yamaguchi T. Effect of body mass index on short-term outcomes of patients undergoing laparoscopic resection for colorectal cancer: a single institution experience in Japan. Surg Laparosc Endosc Percutan Tech. 2011;21:409-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 80. | Senagore AJ, Delaney CP, Madboulay K, Brady KM, Fazio VW. Laparoscopic colectomy in obese and nonobese patients. J Gastrointest Surg. 2003;7:558-561. [PubMed] [Cited in This Article: ] |
| 81. | Canedo J, Pinto RA, Regadas S, Regadas FS, Rosen L, Wexner SD. Laparoscopic surgery for inflammatory bowel disease: does weight matter? Surg Endosc. 2010;24:1274-1279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | Köckerling F, Rose J, Schneider C, Scheidbach H, Scheuerlein H, Reymond MA, Reck T, Konradt J, Bruch HP, Zornig C. Laparoscopic colorectal anastomosis: risk of postoperative leakage. Results of a multicenter study. Laparoscopic Colorectal Surgery Study Group (LCSSG). Surg Endosc. 1999;13:639-644. [PubMed] [Cited in This Article: ] |
| 83. | Kirchhoff P, Dincler S, Buchmann P. A multivariate analysis of potential risk factors for intra- and postoperative complications in 1316 elective laparoscopic colorectal procedures. Ann Surg. 2008;248:259-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 84. | Krane MK, Allaix ME, Zoccali M, Umanskiy K, Rubin MA, Villa A, Hurst RD, Fichera A. Preoperative infliximab therapy does not increase morbidity and mortality after laparoscopic resection for inflammatory bowel disease. Dis Colon Rectum. 2013;56:449-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 85. | Garfinkle R, Abou-Khalil J, Morin N, Ghitulescu G, Vasilevsky CA, Gordon P, Demian M, Boutros M. Is There a Role for Oral Antibiotic Preparation Alone Before Colorectal Surgery? ACS-NSQIP Analysis by Coarsened Exact Matching. Dis Colon Rectum. 2017;60:729-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 86. | Yost MT, Jolissaint JS, Fields AC, Whang EE. Mechanical and Oral Antibiotic Bowel Preparation in the Era of Minimally Invasive Surgery and Enhanced Recovery. J Laparoendosc Adv Surg Tech A. 2018;28:491-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Morris MS, Graham LA, Chu DI, Cannon JA, Hawn MT. Oral Antibiotic Bowel Preparation Significantly Reduces Surgical Site Infection Rates and Readmission Rates in Elective Colorectal Surgery. Ann Surg. 2015;261:1034-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 88. | Hata H, Yamaguchi T, Hasegawa S, Nomura A, Hida K, Nishitai R, Yamanokuchi S, Yamanaka T, Sakai Y. Oral and Parenteral Versus Parenteral Antibiotic Prophylaxis in Elective Laparoscopic Colorectal Surgery (JMTO PREV 07-01): A Phase 3, Multicenter, Open-label, Randomized Trial. Ann Surg. 2016;263:1085-1091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 89. | Ikeda A, Konishi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Y, Akiyoshi T, Yamaguchi T. Randomized clinical trial of oral and intravenous versus intravenous antibiotic prophylaxis for laparoscopic colorectal resection. Br J Surg. 2016;103:1608-1615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 90. | Ris F, Liot E, Buchs NC, Kraus R, Ismael G, Belfontali V, Douissard J, Cunningham C, Lindsey I, Guy R. Multicentre phase II trial of near-infrared imaging in elective colorectal surgery. Br J Surg. 2018; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |