Published online Jun 28, 2015. doi: 10.3748/wjg.v21.i24.7514
Peer-review started: November 17, 2014
First decision: December 26, 2014
Revised: February 6, 2015
Accepted: March 18, 2015
Article in press: March 19, 2015
Published online: June 28, 2015
AIM: To analyze a modified staging system utilizing lymph node ratio (LNR) in patients with esophageal squamous cell carcinoma (ESCC).
METHODS: Clinical data of 2011 patients with ESCC who underwent surgical resection alone between January 1995 and June 2010 at the Cancer Hospital of Shantou University Medical College were reviewed. The LNR, or node ratio (Nr) was defined as the ratio of metastatic LNs ompared to the total number of resected LNs. Overall survival between groups was compared with the log-rank test. The cutoff point of LNR was established by grouping patients with 10% increment in Nr, and then combining the neighborhood survival curves using the log-rank test. A new TNrM staging system, was constructed by replacing the American Joint Committee on Cancer (AJCC) N categories with the Nr categories in the new TNM staging system. The time-dependent receiver operating characteristic curves were used to evaluate the predictive performance of the seventh edition AJCC staging system and the TNrM staging system.
RESULTS: The median number of resected LNs was 12 (range: 4-44), and 25% and 75% interquartile rangeswere8 and 16. Patients were classified into four Nr categories with distinctive survival differences (Nr0: LNR = 0; Nr1: 0% < LNR ≤ 10%; Nr2: 10% < LNR ≤ 20%; and Nr3: LNR > 20%). From N categories to Nr categories, 557 patients changed their LN stage. The median survival time (MST) for the four Nr categories (Nr0-Nr3) was 155.0 mo, 39.0 mo, 28.0 mo, and 19.0 mo, respectively, and the 5-year overall survival was 61.1%, 41.1%, 33.0%, and 22.9%, respectively (P < 0.001). Overall survival was significantly different for the AJCC N categories when patients were subgrouped into 15 or more vs fewer than 15 examined nodes, except for the N3 category (P = 0.292). However, overall survival was similar when the patients in all four Nr categories were subgrouped into 15 or more vs fewer than 15 nodes. Using the time-dependent receiver operating characteristic, we found that the Nr category and TNrM stage had higher accuracy in predicting survival than the AJCC N category and TNM stage.
CONCLUSION: A staging system based on LNR may have better prognostic stratification of patients with ESCC than the current TNM system, especially for those undergoing limited lymphadenectomy.
Core tip: The lymph node ratio (LNR) or node ratio (Nr) is an independent prognostic factor in esophageal cancer patients. In the current study, we evaluated an LNR-based staging system in patients with esophageal squamous cell carcinoma (ESCC) and compared it with the seventh edition American Joint Committee on Cancer (AJCC) staging system. We propose optimal Nr categories for ESCC, and demonstrated that a TNrM staging system bases on LNR may have better prognostic stratification of patients than the AJCC staging system. The application of this new staging system may aid oncologists in improved prediction of prognosis.
- Citation: Chen SB, Weng HR, Wang G, Zou XF, Liu DT, Chen YP, Zhang H. Lymph node ratio-based staging system for esophageal squamous cell carcinoma. World J Gastroenterol 2015; 21(24): 7514-7521
- URL: https://www.wjgnet.com/1007-9327/full/v21/i24/7514.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i24.7514
Esophageal cancer is the seventh leading cause of cancer mortality worldwide[1]. An estimated 16980 people will be diagnosed and 15590 people will eventually die of their disease in the United States in 2015[2]. Surgical resection is the mainstay of therapy for patients with resectable disease[3]. Varied types of surgical procedures (transhiatal, left thoracotomy, right thoracotomy, and minimally invasive surgery) are acceptable for esophagectomy in patients with resectable disease, leading to a significant variability in the extent of lymphadenectomy and the number of lymph nodes (LNs) resected.
The American Joint Committee on Cancer (AJCC) revised and published the seventh edition TNM staging system for esophageal cancer in 2010. This staging system presents a significant improvement for N categories by stratifying patients according to the numbers of positive LNs[4]. However, this system does not specify the adequate number of examined LNs for an accurate nodal staging. In patients with an inadequate number of LNs being examined, stage migration may occur, and lead to understaging of the disease[5].
The lymph node ratio (LNR), or node ratio (Nr), is defined as the ratio of metastatic LNs compared with the total number of resected LNs. Most previous studies have found that LNR is another independent prognostic factor in esophageal cancer patients[6-20]. However, fewer studies have examined whether the LNR has an improved ability to predict survival compared with the absolute number of positive nodes as stratified by the new staging criteria. Moreover, the optimal cutoff points of LNR are still controversial. The differences in study sizes, inclusion criteria, and statistical methods lead to different results.
In a previous study[21], we have found that the N categories of the seventh AJCC staging system do not well represent survival characteristics in ESCC patients in China. We used the data from this study to propose optimal Nr categories, and compared the predictive ability of these Nr categories with the N categories in the current study. We further evaluated the predictive performance of a TNrM staging system compared with the current TNM staging system.
This study was approved by the Ethics Committee of the Cancer Hospital of Shantou University Medical College (CH-SUMC). A total of 3375 patients with esophageal carcinoma underwent esophagectomy in CH-SUMC from January 1995 to June 2010, and we enrolled patients with ESCC with neither neoadjuvant nor adjuvant therapy(esophagectomy alone).
The surgical procedure has been described in our previous report and is summarized below[21]. A transthoracic en bloc esophagectomy was performed via a left or right thoracotomy. A standard two-field lymphadenectomy (abdominal and thoracic lymphadenectomy) was performed in all patients. When patients underwent right thoracotomy, the paratracheal, left and right recurrent laryngeal nerve LNs were also resected. Cervical lymphadenectomy was not systematically undertaken.
All operations were performed or closely supervised by two senior surgeons (Chen YP and Yang JS), and all resection specimens, including the LNs, were assessed by two expert pathologists (Wu MY and Tian DP) in a standardized fashion.
Patients were followed with a clinical examination every 3 mo for the first year, every 6 mo for the second year, and every 6-12 mo thereafter. The routine examination during the follow-up included a clinical evaluation, blood biochemistry examination, ultrasonography, and X-ray examination. Computed tomography was performed every 6 mo. Endoscopic examinations were performed when necessary. Follow-up was continued up to June 2011 or until death, whichever occurred earlier.
The National Comprehensive Cancer Network (NCCN) guidelines (version 1, 2014) recommend at least 15 LNs to be removed for adequate nodal staging for patients undergoing surgical resection without neoadjuvant therapy. We stratified all patients into two groups for analysis: adequate lymphadenectomy (≥15 LNs) and inadequate lymphadenectomy (< 15 LNs).
Overall survivals between groups were compared with the log-rank test. The cutoff points of LNR were established by grouping patients into 10% increments in Nr, and then combining the neighborhood survival curves using the log-rank test. A new TNrM staging system was constructed by replacing the AJCC N categories with the Nr categories in the new AJCC staging system.
The time-dependent receiver operating characteristic (ROC) is an extension of the classic ROC, which permits an evaluation of the diagnostic performance of biomarkers at all time points of interest[22,23]. To assess the predictive ability of the TNM staging system and TNrM staging system, we compared the time-dependent ROC curves for these two staging systems and used the area under the ROC curve (AUC) as the criterion. A larger AUC indicated better predictability of time to event. An AUC of 0.5 indicated no predictive ability, whereas a value of 1 represented perfect predictive ability.
Statistical analysis was performed using SPSS version 13.0 software (SPSS Inc., Chicago, IL, United States), while time-dependent ROC analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria). All statistical tests were performed two-sided, and P < 0.05 was considered to be statistically significant.
The patient characteristics have been summarized in our previous report[21]. A total of 2011 patients with a median age of 55 years (range: 30-82 years) were enrolled in this study, including 1456 male and 555 female patients. The R1 resection rate was 4.1% (83/2011), and R2 resection rate was 3.1% (63/2011). The overall postoperative 30-d mortality was 1.2% (24/2011). The median number of resected LNs was 12 (range: 4-44), and 25% and 75% interquartile ranges were 8 and 16.
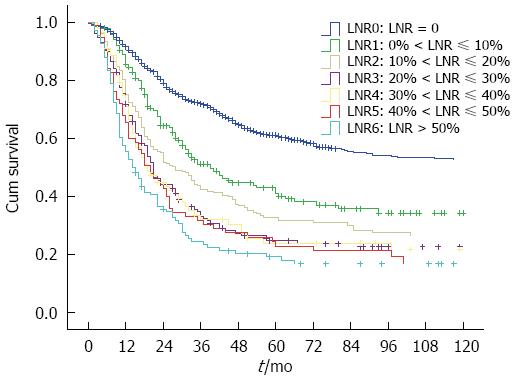
We grouped all patients into 10% increments in Nr to search for possible cutoff points. Only 108 patients had LNR > 50%, and they were taken into a separate group. The patients with LNR > 50% were taken into a group as the small patient numbers (total 108 patients). So, seven Nr stages (LNR = 0, 0% < LNR ≤ 10%, 10% < LNR ≤ 20%, 20% < LNR ≤ 30%, 30% < LNR ≤ 40%, 40% < LNR ≤ 50%, LNR > 50%) were established for analysis (Figure 1). We combined the neighborhood survival curves using the log-rank test, and patients were stratified into four Nr groups (Nr0 to Nr3), based on the following intervals: Nr0: LNR = 0; Nr1: 0% < LNR ≤ 10%; Nr2: 10% < LNR ≤ 20%; and Nr3: LNR > 20%.
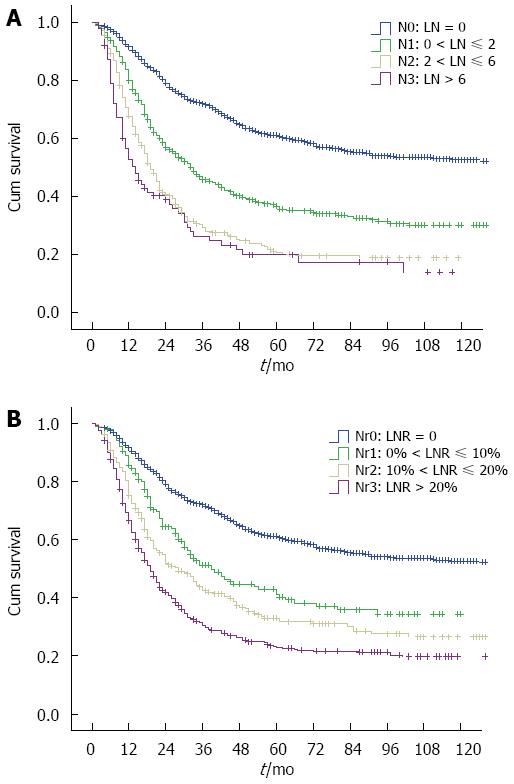
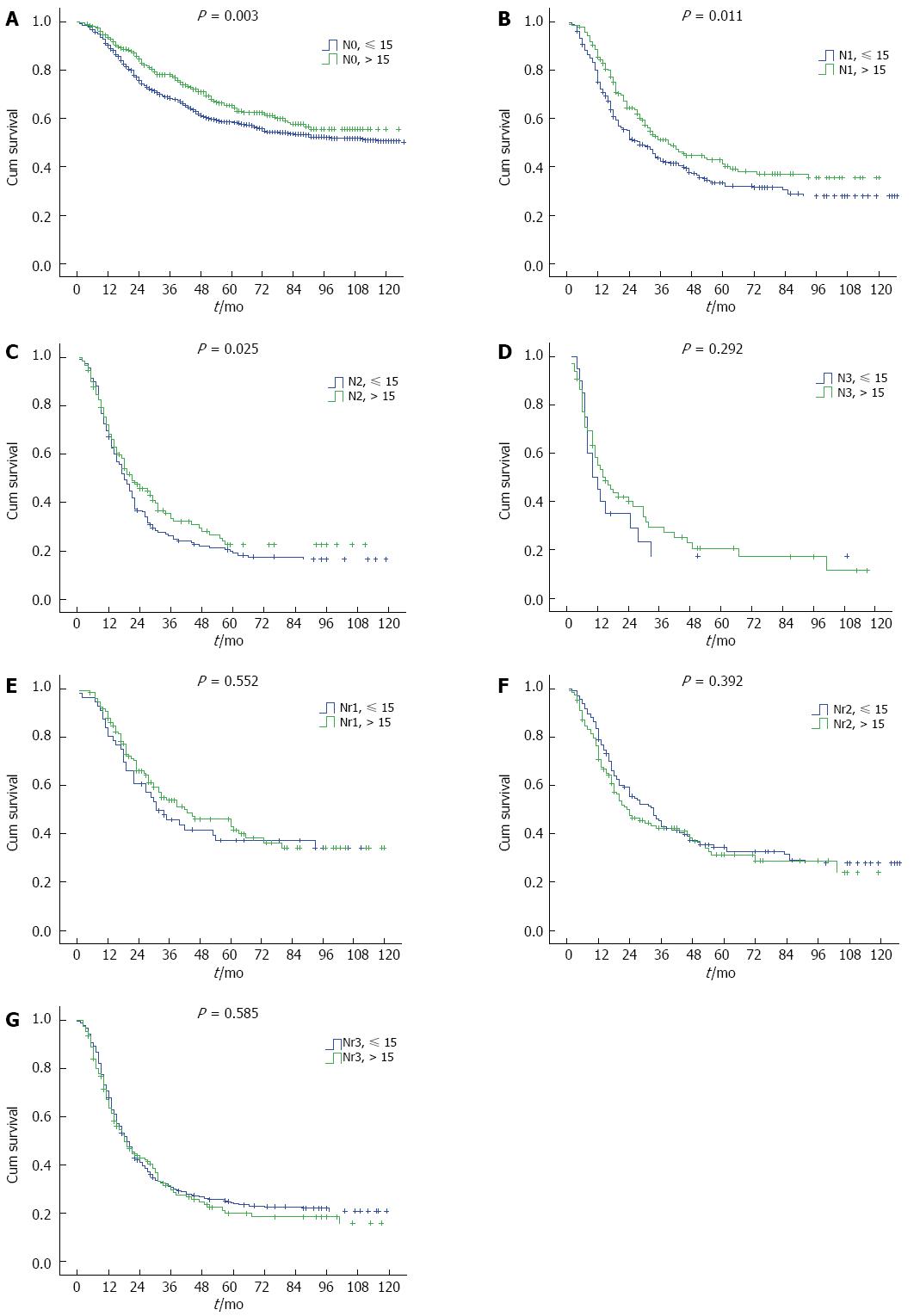
From N categories to Nr categories, 557 patients change their LN stage (Table 1). The median survival time (MST) for AJCC N categories (N0-N3) was 155.0 mo, 33.0 mo, 19.0 mo, and 14.0 mo, respectively, and the 5-year overall survival was 61.1%, 36.6%, 20.7%, and 20.0%, respectively (P < 0.001). However, the survival difference was not significant for N2 vs N3 category (P = 0.159) in a subgroup analysis (Figure 2A). When patients in the N0-N3 categories were stratified into subgroups of adequate lymphadenectomy and inadequate lymphadenectomy, those with adequate lymphadenectomy had significantly better survival than those with inadequate lymphadenectomy (P < 0.05, Figure 3A-C), except for N3 category (P = 0.292, Figure 3D). However, the patient number in the N3 category was only 86. The MST for the four Nr categories (Nr0-Nr3) was 155.0 mo, 39.0 mo, 28.0 mo, and 19.0 mo, respectively, and the 5-year overall survival was 61.1%, 41.1%, 33.0%, and 22.9%, respectively (P < 0.001). The survival differences were significant in a separate subgroup analysis (P value for Nr0 vs Nr1: < 0.001, Nr1 vs Nr2: 0.021, Nr2 vs Nr3: 0.001;Figure 2B). No significant difference was observed in the 5-year overall survival when the Nr1, Nr2 and Nr3 categories were stratified into subgroups of adequate lymphadenectomy and inadequate lymphadenectomy (P > 0.05;Figure 3E-G).
| Nr0 | Nr1 | Nr2 | Nr3 | Total | |
| N0 | 1136 | 0 | 0 | 0 | 1136 |
| N1 | 0 | 187 | 213 | 96 | 496 |
| N2 | 0 | 11 | 46 | 236 | 293 |
| N3 | 0 | 0 | 1 | 85 | 86 |
| Total | 1136 | 198 | 260 | 417 | 2011 |
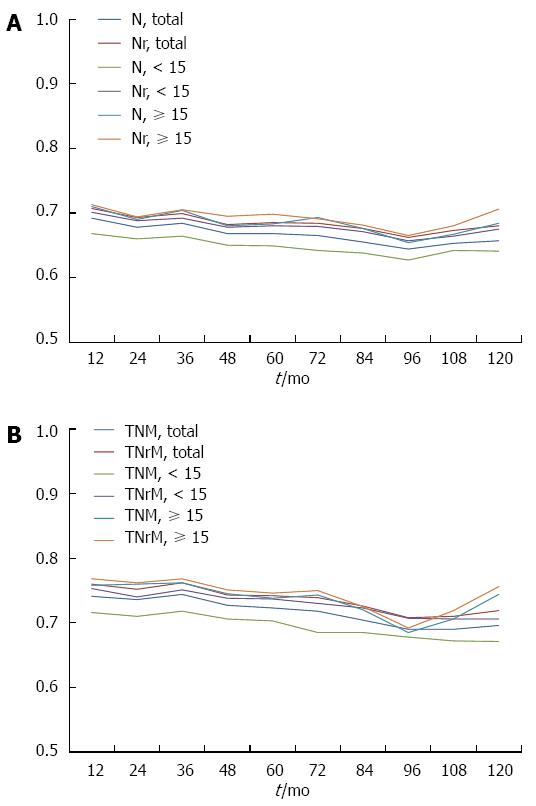
The predictive ability of the current TNM staging system and the TNrM staging system was further evaluated by using time-dependent ROC, which was performed by estimating the value of AUC according to time-dependent sensitivity and specificity. The AUCs for Nr categories were higher than those for N categories (Figure 4A), which indicated that Nr categories had better predictive value than N categories. The TNrM stage also had higher accuracy in predicting survival than the AJCC TNM stage (Figure 4B).We further evaluated the predictive ability of these two staging systems in the subgroups of patient with adequate lymphadenectomy and inadequate lymphadenectomy. We also found in both of these two subgroups that the Nr categories and TNrM stage had a higher accuracy in predicting survival than the AJCC N categories and TNM stage (Figure 4).
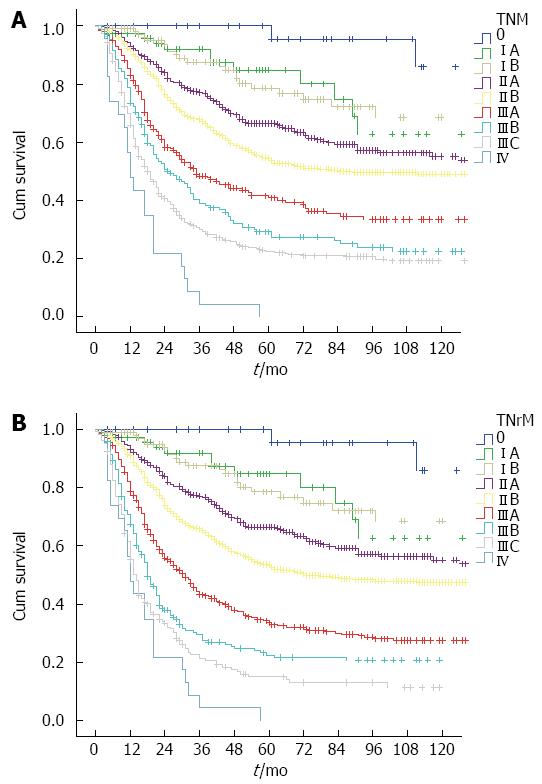
The 5-year overall survival for the current TNM staging system was: stage 0 100%, stage IA 84.8%, stage IB 78.6%, stage IIA 66.5%, stage IIB 53.4%, stage IIIA 33.6%, stage IIIB 22.4%, stage IIIC 15%, and stage IV 0%. For the TNrM staging system, 5-year overall survival was as follows: stage 0 100%, stage IA 84.8%, stage IB 78.6%, stage IIA 66.5%, stage IIB 54.5%, stage IIIA 40.8%, stage IIIB 29.2%, stage IIIC 22.2%, and stage IV 0%. The cumulative survival curves according to these two staging systems are shown in Figure 5. All survival curves were well separated except for stage IA vs IB (P = 0.922).
An accurate staging classification for cancer, according to guidelines that are internationally accepted among surgeons, oncologists, and other physicians, is crucial[13]. An ideal cancer staging system should not only provide an indication of prognosis and a framework for treatment decisions, but should also allow evaluation of treatment with meaningful comparisons between patient cohorts across different institutions and locations[24,25].
The AJCC TNM staging system is now the most commonly used system for esophageal cancer to classify the severity of disease. In 2010, the AJCC published the latest edition of the TNM system for esophageal cancer. The most notable change for this new staging system is the reclassification of N categories by grouping patients based on the numbers of metastatic LNs, which may have greater prognostic importance for esophageal cancer patients[26]. However, although this system advocates as extensive a lymphadenectomy as possible, it does not specify the adequate number of resected LNs for accurate nodal staging.
The extent of lymphadenectomy for esophageal cancer is still controversial[6-8,27]. There are various types of surgical procedures for esophagectomy performed in different institutions, leading to a significant variability in the number of LNs examined. Previous studies have shown that the total number of LNs resected is an independent prognostic predictor for esophageal cancer patients undergoing surgery[27,28]. Because the number of positive nodes is confounded by the total number of nodes examined, nodal categorization based on only the numbers of positive nodes cannot accurately classify all nodal status when insufficient lymphadenectomy is performed. Our data show that when patients are categorized by the AJCC N categories, those with ≥ 15 nodes examined have significantly better survival than those with < 15 nodes examined at the same N stage. This indicates that insufficient lymphadenectomy leads to understaging of the disease. However, further studies are required to specify the minimum number of examined nodes to maximize survival.
LNR has been found to be another independent prognostic factor for esophageal cancer patients after surgery[6-20], and may stratify survival even better than the AJCC N category for certain cohorts of patients[15]. However, most previous studies were with small patient cohorts, and seldom specified what calculations were performed to retrieve the optimal cutoff point[12]. Moreover, few studies were concerned on the predictive performance of LNR in esophageal cancer patients with adequate lymphadenectomy and inadequate lymphadenectomy.
Our study is believed to be the largest ever single-center patient cohort of ESCC to evaluate the predictive ability of LNR. We propose optimal Nr categories for ESCC (Nr0: LNR = 0; Nr1: 0% < LNR ≤ 10%; Nr2: 10% < LNR ≤ 20%; and Nr3: LNR > 20%), which are different from previous studies[6-20]. We found that the survival differences were significant in the four Nr categories in a separate subgroup analysis, while no survival difference was observed for the AJCC N categories of N2 vs N3 (P = 0.159). We also found that the use of Nr categories significantly reduced the range of overall survival inpatients with adequate lymphadenectomy and inadequate lymphadenectomy compared with that of the N categories, suggesting that the Nr categories are a better measure of the extent of regional LN involvement than N categories, particularly in patients with inadequate lymphadenectomy.
Few studies have compared the predictive performance of a TNrM staging system with the new AJCC staging system. Hou et al[12] found that when replacing the N categories with Nr categories in the new AJCC staging system, the survival rate could be easily distinguished between patients. In the current study, we evaluated the predictive performance of a TNrM staging system for the first time using the time-dependent ROC curves, and found that Nr categories and TNrM stages predicted survival better than the N categories and TNM stage. We can also confirmed the same result in the subgroups of patients with ≥ 15 nodes and those with < 15 nodes examined, indicating that Nr categories and TNrM stage may be a better discriminator. However, more studies are required to confirm these findings before firm recommendations can be made.
Our study had some limitations. First, this new TNrM staging system may be improved with better T, Nr, M classifications or other factors, such as a subdivision of T1 cancer into T1a and T1b. However, as the AJCC TNM staging system is now the most commonly used system for esophageal cancer, a TNrM staging system based on the TNM staging system may have wider application. Second, this was a single-institution, retrospective study. Whether this new TNrM system is applicable in another data set or patients with adenocarcinoma needs further validation. Third, most of the patients underwent a standard two-field lymphadenectomy in this study. The number of LNs examined in each patient was limited (median 12 per case). Thus, it may not be optimal for cohorts of patients in which more extensive lymphadenectomy is performed. More extensive lymphadenectomy results in a greater number of uninvolved nodes being removed, thereby driving down the LNR[29,30], so modification of the Nr intervals may be needed for such cohorts. However, our staging system may be more suitable for patients who undergo esophagectomy with limited lymphadenectomy. Finally, whether the TNrM staging system is applicable to patients with neoadjuvant or adjuvant therapy needs further research, as all of our patients underwent surgical resection alone.
In conclusion, we propose optimal Nr categories (Nr0: LNR = 0; Nr1: 0% < LNR ≤ 10%; Nr2: 10% < LNR ≤ 20%; and Nr3: LNR > 20%) for ESCC, and demonstrated that a staging system based on LNR may have better prognostic stratification of patients with ESCC than the current TNM staging system, especially for those undergoing less extensive lymphadenectomy. Further studies are required to confirm our results.
We thank Dr. Jeremy Ganz for proofreading the manuscript.
The seventh edition American Joint Committee on Cancer (AJCC) staging system for esophageal cancer was published in 2010. This staging system presents a significant improvement for N categories by stratifying patients according to the numbers of positive lymph nodes (LNs). However, stage migration may occur in patients with an inadequate number of LNs being examined in this new system. A staging system based on the lymph node ratio (LNR) may be a compensation for the AJCC staging system.
Previous studies have found that LNR is an independent prognostic factor in esophageal cancer patients. However, fewer studies have examined whether the LNR has an improved ability to predict survival compared with the new staging criteria. Moreover, the optimal cutoff points of LNR are still controversial. The authors reported one of the largest ever single-center patient cohorts of esophageal squamous cell carcinoma (ESCC) to evaluate the predictive ability of LNR, and further evaluate the predictive performance of a TNrM staging system compared with the current TNM staging system.
The authors propose optimal node ratio (Nr) categories for ESCC in this study (Nr0: LNR = 0; Nr1: 0% < LNR ≤ 10%; Nr2: 10% < LNR ≤ 20%; and Nr3: LNR > 20%), which are different from previous studies. They also demonstrate that a staging system based on LNR may have better prognostic stratification of patients with ESCC than the current TNM staging system, especially for those undergoing less extensive lymphadenectomy.
The use of this new TNrM staging system in their study may aid oncologists to improve prediction of survival, making treatment decisions, and comparing cohorts of patients, especially those undergoing more limited lymphadenectomy.
A TNrM staging system bases on LNR may have better prognostic stratification of patients with ESCC than the AJCC TNM staging system, especially for those undergoing limited lymphadenectomy.
This is a good paper which reports a large series of patients who underwent esophagectomy for SCC without neoadjuvant treatment. The data analysis appears sound and a good case is made for using the lymph node ratio to determine prognosis.
P- Reviewer: Tsuburaya A, Watson DI S- Editor: Yu J L- Editor: Kerr C E- Editor: Zhang DN
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25175] [Article Influence: 1936.5] [Reference Citation Analysis (3)] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9172] [Cited by in F6Publishing: 9815] [Article Influence: 1090.6] [Reference Citation Analysis (0)] |
| 3. | Castoro C, Scarpa M, Cagol M, Ruol A, Cavallin F, Alfieri R, Zanchettin G, Rugge M, Ancona E. Nodal metastasis from locally advanced esophageal cancer: how neoadjuvant therapy modifies their frequency and distribution. Ann Surg Oncol. 2011;18:3743-3754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Rice TW, Rusch VW, Apperson-Hansen C, Allen MS, Chen LQ, Hunter JG, Kesler KA, Law S, Lerut TE, Reed CE. Worldwide esophageal cancer collaboration. Dis Esophagus. 2009;22:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 271] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 5. | Wang J, Dang P, Raut CP, Pandalai PK, Maduekwe UN, Rattner DW, Lauwers GY, Yoon SS. Comparison of a lymph node ratio-based staging system with the 7th AJCC system for gastric cancer: analysis of 18,043 patients from the SEER database. Ann Surg. 2012;255:478-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 6. | Bogoevski D, Onken F, Koenig A, Kaifi JT, Schurr P, Sauter G, Izbicki JR, Yekebas EF. Is it time for a new TNM classification in esophageal carcinoma? Ann Surg. 2008;247:633-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, Wisnivesky JP. Prognostic significance of the number of lymph node metastases in esophageal cancer. J Am Coll Surg. 2008;206:239-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Kelty CJ, Kennedy CW, Falk GL. Ratio of metastatic lymph nodes to total number of nodes resected is prognostic for survival in esophageal carcinoma. J Thorac Oncol. 2010;5:1467-1471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Dhar DK, Hattori S, Tonomoto Y, Shimoda T, Kato H, Tachibana M, Matsuura K, Mitsumoto Y, Little AG, Nagasue N. Appraisal of a revised lymph node classification system for esophageal squamous cell cancer. Ann Thorac Surg. 2007;83:1265-1272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Kunisaki C, Akiyama H, Nomura M, Matsuda G, Otsuka Y, Ono HA, Shimada H. Developing an appropriate staging system for esophageal carcinoma. J Am Coll Surg. 2005;201:884-890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Wilson M, Rosato EL, Chojnacki KA, Chervoneva I, Kairys JC, Cohn HE, Rosato FE, Berger AC. Prognostic significance of lymph node metastases and ratio in esophageal cancer. J Surg Res. 2008;146:11-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Hou X, Wei JC, Xu Y, Luo RZ, Fu JH, Zhang LJ, Lin P, Yang HX. The positive lymph node ratio predicts long-term survival in patients with operable thoracic esophageal squamous cell carcinoma in China. Ann Surg Oncol. 2013;20:1653-1659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Wijnhoven BP, Tran KT, Esterman A, Watson DI, Tilanus HW. An evaluation of prognostic factors and tumor staging of resected carcinoma of the esophagus. Ann Surg. 2007;245:717-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Fox M, Farmer R, Scoggins CR, McMasters KM, Martin RC. Lymph node ratio is a significant predictor of disease-specific mortality in patients undergoing esophagectomy for cancer. Am Surg. 2012;78:528-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 15. | Bhamidipati CM, Stukenborg GJ, Thomas CJ, Lau CL, Kozower BD, Jones DR. Pathologic lymph node ratio is a predictor of survival in esophageal cancer. Ann Thorac Surg. 2012;94:1643-1651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Feng JF, Huang Y, Chen L, Zhao Q. Prognostic analysis of esophageal cancer in elderly patients: metastatic lymph node ratio versus 2010 AJCC classification by lymph nodes. World J Surg Oncol. 2013;11:162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Hsu WH, Hsu PK, Hsieh CC, Huang CS, Wu YC. The metastatic lymph node number and ratio are independent prognostic factors in esophageal cancer. J Gastrointest Surg. 2009;13:1913-1920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Mariette C, Piessen G, Briez N, Triboulet JP. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg. 2008;247:365-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 315] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 19. | He Z, Wu S, Li Q, Lin Q, Xu J. Use of the metastatic lymph node ratio to evaluate the prognosis of esophageal cancer patients with node metastasis following radical esophagectomy. PLoS One. 2013;8:e73446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Tan Z, Ma G, Yang H, Zhang L, Rong T, Lin P. Can lymph node ratio replace pn categories in the tumor-node-metastasis classification system for esophageal cancer? J Thorac Oncol. 2014;9:1214-1221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Chen SB, Weng HR, Wang G, Yang JS, Yang WP, Liu DT, Chen YP, Zhang H. Prognostic factors and outcome for patients with esophageal squamous cell carcinoma underwent surgical resection alone: evaluation of the seventh edition of the American Joint Committee on Cancer staging system for esophageal squamous cell carcinoma. J Thorac Oncol. 2013;8:495-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Saha-Chaudhuri P, Heagerty PJ. Non-parametric estimation of a time-dependent predictive accuracy curve. Biostatistics. 2013;14:42-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Song X, Zhou XH, Ma S. Nonparametric receiver operating characteristic-based evaluation for survival outcomes. Stat Med. 2012;31:2660-2675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Zhang J, Niu Z, Zhou Y, Cao S. A comparison between the seventh and sixth editions of the American Joint Committee on Cancer/International Union Against classification of gastric cancer. Ann Surg. 2013;257:81-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Zhang H, Wang W, Diao D, Cheng Y, Song Y, Zhu K, Dang C. Ratio of metastatic to examined lymph nodes, a helpful staging system and independent prognostic factor of esophagogastric junction cancer. PLoS One. 2013;8:e73238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721-1724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 571] [Cited by in F6Publishing: 630] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 27. | Peyre CG, Hagen JA, DeMeester SR, Altorki NK, Ancona E, Griffin SM, Hölscher A, Lerut T, Law S, Rice TW. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg. 2008;248:549-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 364] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 28. | Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable esophageal cancer. J Gastrointest Surg. 2007;11:1384-193; discussion 1384-193;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Nigro JJ, DeMeester SR, Hagen JA, DeMeester TR, Peters JH, Kiyabu M, Campos GM, Oberg S, Gastal O, Crookes PF. Node status in transmural esophageal adenocarcinoma and outcome after en bloc esophagectomy. J Thorac Cardiovasc Surg. 1999;117:960-968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 121] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Yang HX, Xu Y, Fu JH, Wang JY, Lin P, Rong TH. An evaluation of the number of lymph nodes examined and survival for node-negative esophageal carcinoma: data from China. Ann Surg Oncol. 2010;17:1901-1911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |