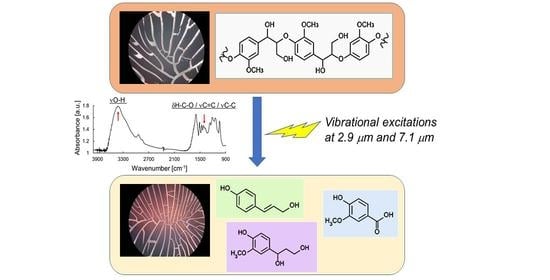
Degradation of Lignin by Infrared Free Electron Laser
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Infrared Free Electron Laser Irradiation
2.3. Synchrotron-Radiation Infrared Microspectroscopy (SR-IRM)
2.4. Electron-Spray Ionization Mass Spectroscopy
2.5. Nuclear Magnetic Resonance Spectroscopy
3. Results
3.1. Irradiation Effect of IR-FEL at Near-Infrared Wavelength
3.2. Irradiation Effect of IR-FEL at Mid-Infrared Wavelengths
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [Green Version]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules 2021, 26, 753. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Current state-of-the-art in ethanol production from lignocellulosic feedstocks. Microbiol. Res. 2020, 240, 126534. [Google Scholar] [CrossRef]
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Ivančić Šantek, M.; Komes, D.; Novak, S.; Šantek, B. Bioethanol Production from Renewable Raw Materials and Its Separation and Purification: A Review. Food Technol. Biotechnol. 2018, 56, 289–311. [Google Scholar] [CrossRef]
- Lee, K.Y.; Shamsuddin, S.R.; Fortea-Verdejo, M.; Bismarck, A. Manufacturing of robust natural fiber preforms utilizing bacterial cellulose as binder. J. Vis. Exp. 2014, 87, 51432. [Google Scholar] [CrossRef] [Green Version]
- Hobisch, M.A.; Zabler, S.; Bardet, S.M.; Zankel, A.; Nypelö, T.; Eckhart, R.; Bauer, W.; Spirk, S. How cellulose nanofibrils and cellulose microparticles impact paper strength-A visualization approach. Carbohydr. Polym. 2021, 254, 117406. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Sefadi, J.S.; Sadiku, E.R.; John, M.J.; Mochane, M.J.; Mtibe, A. Thermoplastic Processing of PLA/Cellulose Nanomaterials Composites. Polymers 2018, 10, 1363. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Cruz-Morales, P.; Zargar, A.; Belcher, M.S.; Pang, B.; Englund, E.; Dan, Q.; Yin, K.; Keasling, J.D. Biofuels for a sustainable future. Cell 2021, 184, 1636–1647. [Google Scholar] [CrossRef]
- Obydenkova, S.V.; Kouris, P.D.; Hensen, E.J.M.; Heeres, H.J.; Boot, M.D. Environmental economics of lignin derived transport fuels. Bioresour. Technol. 2017, 243, 589–599. [Google Scholar] [CrossRef]
- Pineda, A.; Lee, A.F. Heterogeneously catalyzed lignin depolymerization. Appl. Petrochem. Res. 2016, 6, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV Light Blocker-A Review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef]
- Janus, Ł.; Radwan-Pragłowska, J.; Piątkowski, M.; Bogdał, D. Coumarin-Modified, CQDs for Biomedical Applications-Two-Step Synthesis and Characterization. Int. J. Mol. Sci. 2020, 21, 8073. [Google Scholar] [CrossRef]
- Elangovan, S.; Afanasenko, A.; Haupenthal, J.; Sun, Z.; Liu, Y.; Hirsch, A.K.H.; Barta, K. From Wood to Tetrahydro-2-benzazepines in Three Waste-Free Steps: Modular Synthesis of Biologically Active Lignin-Derived Scaffolds. ACS Cent. Sci. 2019, 5, 1707–1716. [Google Scholar] [CrossRef] [Green Version]
- Alzagameem, A.; Khaldi-Hansen, B.E.; Büchner, D.; Larkins, M.; Kamm, B.; Witzleben, S.; Schulze, M. Lignocellulosic Biomass as Source for Lignin-Based Environmentally Benign Antioxidants. Molecules 2018, 23, 2664. [Google Scholar] [CrossRef] [Green Version]
- Santana, C.S.; Aguiar, A. Effect of Lignin-Derived Methoxyphenols in Dye Decolorization by Fenton Systems. Water Air Soil Pollut. 2016, 227, 48. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef] [Green Version]
- Lanzalunga, O.; Bietti, M. Photo- and radiation chemical induced degradation of lignin model compounds. J. Photochem. Photobiol. B Biol. 2000, 56, 85–108. [Google Scholar] [CrossRef]
- Pollegioni, L.; Tonin, F.; Rosini, E. Lignin-degrading enzymes. FEBS J. 2015, 282, 1190–1213. [Google Scholar] [CrossRef]
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; de los Santos, M.H.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C.N.; et al. Laccases: Structure, function, and potential application in water bioremediation. Microb. Cell Fact. 2019, 18, 200. [Google Scholar] [CrossRef]
- Edwards, G.; Logan, R.; Copeland, M.; Reinisch, L.; Davidson, J.; Johnson, B.; Maciunas, R.; Mendenhall, M.; Ossoff, R.; Tribble, J.; et al. Tissue ablation by a free-electron laser tuned to the amide II band. Nature 1994, 371, 416–419. [Google Scholar] [CrossRef]
- Shevchenko, O.A.; Melnikov, A.R.; Tararyshkin, S.V.; Getmanov, Y.V.; Serednyakov, S.S.; Bykov, E.V.; Kubarev, V.V.; Fedin, M.V.; Veber, S.L. Electronic Modulation of THz Radiation at NovoFEL: Technical Aspects and Possible Applications. Materials 2019, 12, 3063. [Google Scholar] [CrossRef] [Green Version]
- Lamb, D.C.; Tribble, J.; Doukas, A.G.; Flotte, T.J.; Ossoff, R.H.; Reinisch, L. Custom designed acoustic pulses. J. Biomed. Opt. 1999, 4, 217–223. [Google Scholar] [CrossRef]
- Elferink, H.; Severijnen, M.E.; Martens, J.; Mensink, R.A.; Berden, G.; Oomens, J.; Rutjes, F.P.J.T.; Rijs, A.M.; Boltje, T.J. Direct Experimental Characterization of Glycosyl Cations by Infrared Ion Spectroscopy. J. Am. Chem. Soc. 2018, 140, 6034–6038. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, D.E.; Morais, C.L.M.; Lima, K.M.G.; Trevisan, J.; Siggel-King, M.R.F.; Craig, T.; Ingham, J.; Martin, D.S.; Heys, K.A.; Kyrgiou, M.; et al. Imaging cervical cytology with scanning near-field optical microscopy (SNOM) coupled with an IR-FEL. Sci. Rep. 2016, 6, 29494. [Google Scholar] [CrossRef] [Green Version]
- Dienst, A.; Casandruc, E.; Fausti, D.; Zhang, L.; Eckstein, M.; Hoffmann, M.; Khanna, V.; Dean, N.; Gensch, M.; Winnerl, S.; et al. Optical excitation of Josephson plasma solitons in a cuprate superconductor. Nat. Mater. 2013, 12, 535–541. [Google Scholar] [CrossRef]
- Carlo, M.J.; Patrick, A.L. Infrared multiple photon dissociation (IRMPD) spectroscopy and its potential for the clinical laboratory. J. Mass Spectrom. Adv. Clin. Lab. 2021, 23, 14–25. [Google Scholar] [CrossRef]
- Kawasaki, T.; Sakai, T.; Zen, H.; Sumitomo, Y.; Nogami, K.; Hayakawa, K.; Yaji, T.; Ohta, T.; Tsukiyama, K.; Hayakawa, T. Cellulose Degradation by Infrared Free Electron Laser. Energy Fuels 2020, 34, 9064–9068. [Google Scholar] [CrossRef]
- Zen, H.; Suphakul, S.; Kii, T.; Masuda, K.; Ohgaki, H. Present status and perspectives of long wavelength free electron lasers at Kyoto University. Phys. Procedia 2016, 84, 47–53. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Sato, I.; Hayakawa, K.; Tanaka, T.; Nakazawa, H.; Yokoyama, K.; Kanno, K.; Sakai, T.; Ishiwata, K.; Enomoto, A.; et al. First lasing of LEBRA FEL at Nihon University at a wavelength of 1.5 μm. Nucl. Instrum. Methods Phys. Res A 2002, 483, 29–33. [Google Scholar] [CrossRef]
- Kuwada-Kusunose, T.; Kusunose, A.; Wakami, M.; Takebayashi, C.; Goto, H.; Aida, M.; Sakai, T.; Nakao, K.; Nogami, K.; Inagaki, M.; et al. Evaluation of irradiation effects of near-infrared free-electron-laser of silver alloy for dental application. Lasers Med. Sci. 2017, 32, 1349–1355. [Google Scholar] [CrossRef]
- Sakai, T.; Hayakawa, K.; Tanaka, T.; Hayakawa, Y.; Nogami, K.; Sei, N. Evaluation of Bunch Length by Measuring Coherent Synchrotron Radiation with a Narrow-Band Detector at LEBRA. Condens. Matter 2020, 5, 34. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Apostolidis, P.; Gard, W.; van de Ven, M.; Erkens, S.; Jing, R. Chemical and Rheological Evaluation of Aged Lignin-Modified Bitumen. Materials 2019, 12, 4176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerbin, E.; Rivière, G.N.; Foulon, L.; Frapart, Y.M.; Cottyn, B.; Pernes, M.; Marcuello, C.; Godon, B.; Gainvors-Claisse, A.; Crônier, D.; et al. Tuning the functional properties of lignocellulosic films by controlling the molecular and supramolecular structure of lignin. Int. J. Biol. Macromol. 2021, 181, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Yaji, T.; Yamamoto, Y.; Ohta, T.; Kimura, S. A new beamline for infrared microscopy in the SR center of Ritsumeikan University. Infrared Phys. Technol. 2008, 51, 397–399. [Google Scholar] [CrossRef]
- Goddard, T.D.; Kneller, D.G. SPARKY 3; University of California: San Francisco, CA, USA, 2006. [Google Scholar]
- Ralph, S.A.; Ralph, J.; Landucci, L.L. NMR Database of Lignin and Cell Wall Model Compounds. 2009. Available online: https://www.glbrc.org/data-and-tools/glbrc-research-tools/nmr-database-lignin-and-cell-wall-model-compounds (accessed on 1 May 2022).
- Matsubara, M.; Osada, F.; Nakajima, M.; Imai, T.; Nishimura, K.; Oyama, T.; Tsukiyama, K. Isomerization and dissociation of 2,3-DHF induced by infrared free electron laser. J. Photochem. Photobiol. A 2016, 322, 53–59. [Google Scholar] [CrossRef]
- Zavalin, A.; Hachey, D.L.; Sundaramoorthy, M.; Banerjee, S.; Morgan, S.; Feldman, L.; Tolk, N.; Piston, D.W. Kinetics of a collagen-like polypeptide fragmentation after mid-IR free-electron laser ablation. Biophys. J. 2008, 95, 1371–1381. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, T.; Sato, A.; Tominaga, Y.; Suzuki, Y.; Oyama, T.; Tadokoro, M.; Tsukiyama, K.; Nokihara, K.; Zen, H. Photo-Modification of Melanin by a Mid-infrared Free-electron Laser. Photochem. Photobiol. 2019, 95, 946–950. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H.-O.; Cho, Y.-J.; Kim, J.; Chun, J.; Choi, J.; Lee, Y.; Jung, W.H. A Vanillin Derivative Causes Mitochondrial Dysfunction and Triggers Oxidative Stress in Cryptococcus neoformans. PLoS ONE 2014, 9, e89122. [Google Scholar] [CrossRef] [Green Version]
- Lubbers, R.J.M.; Dilokpimol, A.; Nousiainen, P.A.; Cioc, R.C.; Visser, J.; Bruijnincx, P.C.A.; de Vries, R.P. Vanillic acid and methoxyhydroquinone production from guaiacyl units and related aromatic compounds using Aspergillus niger cell factories. Microb. Cell Fact. 2021, 20, 151. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawasaki, T.; Zen, H.; Sakai, T.; Sumitomo, Y.; Nogami, K.; Hayakawa, K.; Yaji, T.; Ohta, T.; Nagata, T.; Hayakawa, Y. Degradation of Lignin by Infrared Free Electron Laser. Polymers 2022, 14, 2401. https://doi.org/10.3390/polym14122401
Kawasaki T, Zen H, Sakai T, Sumitomo Y, Nogami K, Hayakawa K, Yaji T, Ohta T, Nagata T, Hayakawa Y. Degradation of Lignin by Infrared Free Electron Laser. Polymers. 2022; 14(12):2401. https://doi.org/10.3390/polym14122401
Chicago/Turabian StyleKawasaki, Takayasu, Heishun Zen, Takeshi Sakai, Yoske Sumitomo, Kyoko Nogami, Ken Hayakawa, Toyonari Yaji, Toshiaki Ohta, Takashi Nagata, and Yasushi Hayakawa. 2022. "Degradation of Lignin by Infrared Free Electron Laser" Polymers 14, no. 12: 2401. https://doi.org/10.3390/polym14122401