Designing New Antibacterial Wound Dressings: Development of a Dual Layer Cotton Material Coated with Poly(Vinyl Alcohol)_Chitosan Nanofibers Incorporating Agrimonia eupatoria L. Extract
Abstract
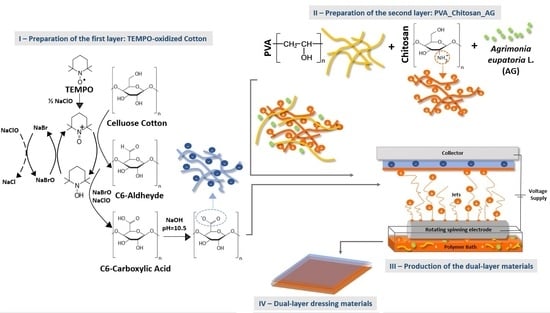
:1. Introduction
2. Results and Discussion
2.1. Minimum Inhibitory Concentration (MIC)
2.2. Characterization of the Dual-Layer Dressing Materials
2.2.1. Assessment of the Morphologic Features
2.2.2. Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.2.3. Mechanical Strength Behavior
2.2.4. Wetting Studies
2.2.5. Porosity Assessment
2.2.6. Water Vapor Transmission Rates (WVTRs)
2.2.7. Swelling and In Vitro Degradation Studies
2.3. Study of the In Vitro AG Release
2.4. Antibacterial Properties of the Dual-Layer Dressing Materials
2.5. In Vitro Cytotoxicity Assay (MTT)
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Crude AG Extract
3.2.2. Minimum Inhibitory Concentration (MIC)
3.2.3. Fabrication of the Dual-Layer Dressing Materials
3.2.4. Characterization of the Dual-Layer Dressing Materials
Assessment of the Morphologic Features
Fourier Transform Infrared Spectroscopy (ATR-FTIR)
Mechanical Strength Behavior
Wetting Studies
Porosity Assessment
Water Vapor Transmission Rates (WVTR)
Swelling and In Vitro Degradation Studies
3.2.5. Study of the In Vitro AG Release
3.2.6. Antibacterial Properties of the Dual-Layer Dressing Materials
3.2.7. In Vitro Cytotoxicity Assay (MTT)
3.2.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chitrattha, S.; Phaechamud, T. Porous poly(dl-lactic acid) matrix film with antimicrobial activities for wound dressing application. Mater. Sci. Eng. C 2016, 58, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, D.M.; Leite, I.S.; Bukzem, A.; de Oliveira Santos, R.P.; Frollini, E.; Inada, N.M.; Campana-Filho, S.P. Nanostructured electrospun nonwovens of poly(ε-caprolactone)/quaternized chitosan for potential biomedical applications. Carbohydr. Polym. 2018, 186, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Williamson, D.; Harding, K. Wound healing. Medicine 2004, 32, 4–7. [Google Scholar] [CrossRef]
- Bowler, P.G. Wound pathophysiology, infection and therapeutic options. Ann. Med. 2002, 34, 419–427. [Google Scholar] [CrossRef]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for wound healing and infection control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef] [Green Version]
- Pinho, E.; Soares, G. Functionalization of cotton cellulose for improved wound healing. J. Mater. Chem. B 2018, 6, 1887–1898. [Google Scholar] [CrossRef]
- Goh, Y.F.; Shakir, I.; Hussain, R. Electrospun fibers for tissue engineering, drug delivery, and wound dressing. J. Mater. Sci. 2013, 48, 3027–3054. [Google Scholar] [CrossRef]
- Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Mohajeri, A.; Fattahi, A.; Sheervalilou, R.; Zarghami, N. An overview on application of natural substances incorporated with electrospun nanofibrous scaffolds to development of innovative wound dressings. Mini-Reviews Med. Chem. 2017, 18, 414–427. [Google Scholar] [CrossRef]
- Punjataewakupt, A.; Napavichayanun, S.; Aramwit, P. The downside of antimicrobial agents for wound healing. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Duan, X.P.; Li, Y.M.; Yang, D.P.; Long, Y.Z. Electrospun nanofibers for wound healing. Mater. Sci. Eng. C 2017, 76, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.R.; Bernstein, J.M. Chronic wound infection: Facts and controversies. Clin. Dermatol. 2010, 28, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Gizaw, M.; Thompson, J.; Faglie, A.; Lee, S.Y.; Neuenschwander, P.; Chou, S.F. Electrospun fibers as a dressing material for drug and biological agent delivery in wound healing applications. Bioengineering 2018, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Ronca, S.; Mele, E. Electrospun nanofibres containing antimicrobial plant extracts. Nanomaterials 2017, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- El-Hamid, M.A. A new promising target for plant extracts: Inhibition of bacterial quorum sensing. J. Mol. Biol. Biotechnol. 2016, 1, 1–3. [Google Scholar]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.D.; Birdi, T.J. Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med. 2017, 8, 266–275. [Google Scholar] [CrossRef]
- Avci, H.; Gergeroglu, H. Synergistic effects of plant extracts and polymers on structural and antibacterial properties for wound healing. Polym. Bull. 2019, 76, 3709–3731. [Google Scholar] [CrossRef]
- Ghaima, K.K. Antibacterial and wound healing activity of some Agrimonia eupatoria extracts. Baghdad Sci. J. 2013, 10, 152–160. [Google Scholar]
- Pirvu, L. Studies on Agrimoniae herba selective extracts; polyphenols content, antioxidant and antimicrobial potency, MTS test. Acad. Rom. Sci. Ann. Biol. Sci. 2016, 5, 96–107. [Google Scholar]
- Muruzović, M.; Mladenović, K.G.; Stefanović, O.D.; Vasić, S.M.; Čomić, L.R. Extracts of Agrimonia eupatoria L. as sources of biologically active compounds and evaluation of their antioxidant, antimicrobial, and antibiofilm activities. J. Food Drug Anal. 2016, 24, 539–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmani Del Bakhshayesh, A.; Annabi, N.; Khalilov, R.; Akbarzadeh, A.; Samiei, M.; Alizadeh, E.; Alizadeh-Ghodsi, M.; Davaran, S.; Montaseri, A. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Papenburg, B.J.; Bolhuis-Versteeg, L.A.M.; Grijpma, D.W.; Feijen, J.; Wessling, M.; Stamatialis, D. A facile method to fabricate poly(L-lactide) nano-fibrous morphologies by phase inversion. Acta Biomater. 2010, 6, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: Advances, challenges, and future prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef]
- Gomes, A.P.; Mano, J.F.; Queiroz, J.A.; Gouveia, I.C. Layer-by-layer deposition of antibacterial polyelectrolytes on cotton fibres. J. Polym. Environ. 2012, 20, 1084–1094. [Google Scholar] [CrossRef]
- Hadisi, Z.; Nourmohammadi, J.; Nassiri, S.M. The antibacterial and anti-inflammatory investigation of Lawsonia Inermis-gelatin-starch nano-fibrous dressing in burn wound. Int. J. Biol. Macromol. 2018, 107, 2008–2019. [Google Scholar] [CrossRef]
- Tandi, A.; Kaur, T.; Ebinesan, P.R.; Thirugnanam, A.; Mondal, A.K. Drug loaded poly (vinyl alcohol)-cellulose composite hydrogels for wound dressings. In Proceedings of the 8th International Conference on Materials for Advanced Technologies of the Materials Research Society of Singapore & IUMRS & 16th International Conference in Asia (ICMAT2015 & IUMRS-ICA2015), Suntec, Singapore, 28 June–3 July 2015; pp. 2–5. [Google Scholar]
- Trinca, R.B.; Westin, C.B.; da Silva, J.A.F.; Moraes, Â.M. Electrospun multilayer chitosan scaffolds as potential wound dressings for skin lesions. Eur. Polym. J. 2017, 88, 161–170. [Google Scholar] [CrossRef]
- Wang, J.; Planz, V.; Vukosavljevic, B.; Windbergs, M. Multifunctional electrospun nanofibers for wound application—Novel insights into the control of drug release and antimicrobial activity. Eur. J. Pharm. Biopharm. 2018, 129, 175–183. [Google Scholar] [CrossRef]
- Jin, G.; Prabhakaran, M.P.; Kai, D.; Annamalai, S.K.; Arunachalam, K.D.; Ramakrishna, S. Tissue engineered plant extracts as nanofibrous wound dressing. Biomaterials 2013, 34, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, J.K.; Park, S.R.; Chang, Y.H. Preparation of collagen/poly(L-lactic acid) composite material for wound dressing. Macromol. Res. 2007, 15, 205–210. [Google Scholar] [CrossRef]
- Fazli, Y.; Shariatinia, Z.; Kohsari, I.; Azadmehr, A.; Pourmortazavi, S.M. A novel chitosan-polyethylene oxide nanofibrous mat designed for controlled co-release of hydrocortisone and imipenem/cilastatin drugs. Int. J. Pharm. 2016, 513, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Carofiglio, V.E.; Stufano, P.; Bonfrate, V.; Calò, E.; Scarlino, S.; Nitti, P.; Centrone, D.; Cascione, M.; Leporatti, S.; et al. Potential of electrospun poly(3-hydroxybutyrate)/collagen blends for tissue engineering applications. J. Healthc. Eng. 2018, 2018, 6573947. [Google Scholar] [CrossRef]
- Sarheed, O.; Ahmed, A.; Shouqair, D.; Boateng, J. Antimicrobial dressings for improving wound healing. In Wound Healing—New insights into Ancient Challenges; InTech: London, UK, 2016; pp. 373–398. [Google Scholar]
- Arkoun, M.; Daigle, F.; Heuzey, M.C.; Ajji, A. Antibacterial electrospun chitosan-based nanofibers: A bacterial membrane perforator. Food Sci. Nutr. 2017, 5, 865–874. [Google Scholar] [CrossRef]
- Yousefi, I.; Pakravan, M.; Rahimi, H.; Bahador, A.; Farshadzadeh, Z.; Haririan, I. An investigation of electrospun Henna leaves extract-loaded chitosan based nanofibrous mats for skin tissue engineering. Mater. Sci. Eng. C 2017, 75, 433–444. [Google Scholar] [CrossRef]
- Wijesinghe, W.P.S.L.; Mantilaka, M.M.M.G.P.G.; Rajapakse, R.M.G.; Pitawala, H.M.T.G.A.; Premachandra, T.N.; Herath, H.M.T.U.; Rajapakse, R.P.V.J.; Wijayantha, K.G.U. Urea-assisted synthesis of hydroxyapatite nanorods from naturally occurring impure apatite rocks for biomedical applications. RSC Adv. 2017, 7, 24806–24812. [Google Scholar] [CrossRef] [Green Version]
- Yeh, C.C.; Li, Y.T.; Chiang, P.H.; Huang, C.H.; Wang, Y.; Chang, H.I. Characterizing microporous PCL matrices for application of tissue engineering. J. Med. Biol. Eng. 2009, 29, 92–97. [Google Scholar]
- Kurkina, A.V. A method for the assay of total flavonoids in common agrimony herb. Pharm. Chem. J. 2011, 45, 43–46. [Google Scholar] [CrossRef]







| Tensile Strength (MPa) | Young’s Modulus (GPa) | Elongation at Break (%) | Thickness (mm) | |
|---|---|---|---|---|
| TEMPO-oxidized cotton | 36.98 ± 4.13 | 1.82 ± 0.25 | 2.04 ± 0.12 | 0.250 ± 0.011 |
| PVA_CS | 9.83 ± 16.38 | 0.41 ± 0.25 | 4.64 ± 1.40 | 0.051 ± 0.004 |
| PVA_AG_CS | 9.66 ± 1.95 | 0.35 ± 0.09 | 3.03 ± 0.60 | 0.012 ± 0.001 |
| TEMPO-oxidized cotton/PVA_CS | 22.48 ± 4.46 | 0.40 ± 0.14 | 5.80 ± 0.89 | 0.315 ± 0.011 |
| TEMPO-oxidized cotton/PVA_AG_CS | 26.55 ± 1.41 | 0.46 ± 0.11 | 5.95 ± 1.71 | 0.270 ± 0.004 |
| Standard Viability Data a | Experimental Data | ||||
|---|---|---|---|---|---|
| Cell Viability (%) | Toxicity Level | Samples | Cell Viability (%) | Toxicity Level | |
| ≥100 | 0 | TEMPO-oxidized cotton | Day 1 | 88.99 | 1 |
| 75–99 | 1 | Day 3 | 98.50 | 1 | |
| 50–74 | 2 | Day 7 | 92.98 | 1 | |
| 25–49 | 3 | TEMPO-oxidized cotton/PVA_CS | Day 1 | 91.83 | 1 |
| 1–24 | 4 | Day 3 | 105.79 | 0 | |
| 0 | 5 | Day 7 | 89.69 | 1 | |
| TEMPO-oxidized cotton/PVA_AG_CS | Day 1 | 92.78 | 1 | ||
| Day 3 | 100.45 | 0 | |||
| Day 7 | 104.64 | 0 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouro, C.; Dunne, C.P.; Gouveia, I.C. Designing New Antibacterial Wound Dressings: Development of a Dual Layer Cotton Material Coated with Poly(Vinyl Alcohol)_Chitosan Nanofibers Incorporating Agrimonia eupatoria L. Extract. Molecules 2021, 26, 83. https://doi.org/10.3390/molecules26010083
Mouro C, Dunne CP, Gouveia IC. Designing New Antibacterial Wound Dressings: Development of a Dual Layer Cotton Material Coated with Poly(Vinyl Alcohol)_Chitosan Nanofibers Incorporating Agrimonia eupatoria L. Extract. Molecules. 2021; 26(1):83. https://doi.org/10.3390/molecules26010083
Chicago/Turabian StyleMouro, Cláudia, Colum P. Dunne, and Isabel C. Gouveia. 2021. "Designing New Antibacterial Wound Dressings: Development of a Dual Layer Cotton Material Coated with Poly(Vinyl Alcohol)_Chitosan Nanofibers Incorporating Agrimonia eupatoria L. Extract" Molecules 26, no. 1: 83. https://doi.org/10.3390/molecules26010083