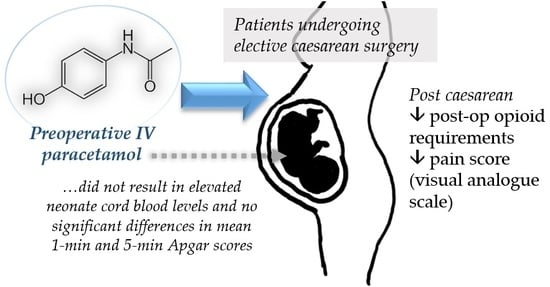
A Meta-Analysis of the Utility of Preoperative Intravenous Paracetamol for Post-Caesarean Analgesia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boerma, T.; Ronsmans, C.; Melesse, D.Y.; Barros, A.J.; Barros, F.C.; Juan, L.; Moller, A.B.; Say, L.; Hosseinpoor, A.R.; Yi, M.; et al. Global epidemiology of use of and disparities in caesarean sections. Lancet 2018, 392, 1341–1348. [Google Scholar] [CrossRef]
- Gin, T.; Ngan-Kee, W.D.; Siu, Y.K.; Stuart, J.C.; Tan, P.E.; Lam, K.K. Alfentanil given immediately before the induction of anesthesia for elective cesarean delivery. Anesth. Analg. 2000, 90, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Kolodny, A.; Courtwright, D.T.; Hwang, C.S.; Kreiner, P.; Eadie, J.L.; Clark, T.W.; Alexander, G.C. The prescription opioid and heroin crisis: A public health approach to an epidemic of addiction. Annu. Rev. Public Health 2015, 36, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.H. Ending the opioid epidemic—A call to action. N. Engl. J. Med. 2016, 375, 2413–2415. [Google Scholar] [CrossRef]
- McNicol, E.D.; Tzortzopoulou, A.; Cepeda, M.S.; Francia, M.B.; Farhat, T.; Schumann, R. Single-dose intravenous paracetamol or propacetamol for prevention or treatment of postoperative pain: A systematic review and meta-analysis. Br. J. Anaesth. 2011, 106, 764–775. [Google Scholar] [CrossRef]
- Toms, L.; McQuay, H.J.; Derry, S.; Moore, R.A. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database Syst. Rev. 2008, 4, CD004602. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.V.; Mehta, V. Paracetamol: Mechanisms and updates. Contin. Educ. Anaesth. Crit. Care Pain 2013, 14, 153–158. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Altenau, B.; Crisp, C.C.; Devaiah, C.G.; Lambers, D.S. Randomized controlled trial of intravenous acetaminophen for postcesarean delivery pain control. Am. J. Obstet. Gynecol. 2017, 217, 362.e1–362.e6. [Google Scholar] [CrossRef]
- Ayatollahi, V.; Faghihi, S.; Behdad, S.; Heiranizadeh, N.; Baghianimoghadam, B. Effect of preoperative administration of intravenous paracetamol during cesarean surgery on hemodynamic variables relative to intubation, postoperative pain and neonatal apgar. Acta Clin. Croat. 2014, 53, 272–278. [Google Scholar]
- Hassan, H.I. Perioperative analgesic effects of intravenous paracetamol: Preemptive versus preventive analgesia in elective cesarean section. Anesth. Essays Res. 2014, 8, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Ozmete, O.; Bali, C.; Cok, O.Y.; Ergenoglu, P.; Ozyilkan, N.B.; Akin, S.; Kalayci, H.; Aribogan, A. Preoperative paracetamol improves post-cesarean delivery pain management: A prospective, randomized, double-blind, placebo-controlled trial. J. Clin. Anesth. 2016, 33, 51–57. [Google Scholar] [CrossRef]
- Prasanna, A.; Sharma, K. Pre incision analgesia prevents immediate incidental pain after LSCS-randomised blinded study. J. Anaesthesiol. Clin. Pharmacol. 2010, 26, 375–378. [Google Scholar]
- Soltani, G.; Molkizadeh, A.; Amini, S. Effect of intravenous acetaminophen (paracetamol) on hemodynamic parameters following endotracheal tube intubation and postoperative pain in caesarian section surgeries. Anesthesiol. Pain Med. 2015, 5, e30062. [Google Scholar] [CrossRef]
- Towers, C.V.; Shelton, S.; van Nes, J.; Gregory, E.; Liske, E.; Smalley, A.; Mobley, E.; Faircloth, B.; Fortner, K.B. Preoperative cesarean delivery intravenous acetaminophen treatment for postoperative pain control: A randomized double-blinded placebo control trial. Am. J. Obstet. Gynecol. 2018, 218, 353.e1–353.e4. [Google Scholar] [CrossRef]
- Katz, J. Pre-emptive analgesia: Importance of timing. Can. J. Anesth. 2001, 48, 105–114. [Google Scholar] [CrossRef]
- Beyer, K.; Taffé, P.; Halfon, P.; Pittet, V.; Pichard, S.; Haller, G.; Burnand, B.; ADS Study Group. Hypertension and intra-operative incidents: A multicentre study of 125,000 surgical procedures in Swiss hospitals. Anaesthesia 2009, 64, 494–502. [Google Scholar] [CrossRef]
- Apfel, C.C.; Turan, A.; Souza, K.; Pergolizzi, J.; Hornuss, C. Intravenous acetaminophen reduces postoperative nausea and vomiting: A systematic review and meta-analysis. PAIN® 2013, 154, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.S. Potential analgesic mechanisms of acetaminophen. Pain Physician 2009, 12, 269–280. [Google Scholar]
- Singla, N.K.; Parulan, C.; Samson, R.; Hutchinson, J.; Bushnell, R.; Beja, E.G.; Ang, R.; Royal, M.A. Plasma and cerebrospinal fluid pharmacokinetic parameters after single-dose administration of intravenous, oral, or rectal acetaminophen. Pain Pract. 2012, 12, 523–532. [Google Scholar] [CrossRef]
- Ng, Q.X.; Soh, A.Y.; Yeo, W.S. Statistically but not clinically significant? Biomarkers in gastric cancer. Clin. Nutr. 2018, 37, 2292–2293. [Google Scholar] [CrossRef]
- Bauer, A.Z.; Kriebel, D.; Herbert, M.R.; Bornehag, C.G.; Swan, S.H. Prenatal paracetamol exposure and child neurodevelopment: A review. Horm. Behav. 2018, 101, 125–147. [Google Scholar] [CrossRef]




| Author, Year | Country of Origin | Study Design | Study Sample | Type of Anaesthesia | Intervention | Conclusions |
|---|---|---|---|---|---|---|
| Altenau, 2017 [10] | United States | Randomized, placebo-controlled, double-blind trial | n = 104, pregnant women, scheduled for elective caesarean section, Mean Age 29.6 years | Spinal | - IV paracetamol 1 g given within 30 to 60 min of the surgical incision, and every 8 h for 48 h, for a total of 6 doses | - No significant difference in pain scores but significantly reduced postoperative requirement for opioid. |
| Ayatollahi, 2014 [11] | Iran | Randomized, placebo-controlled, double-blind trial | n = 60, pregnant women, ASA class I, scheduled for elective caesarean section, Age 18 to 40 years | General | - IV paracetamol 1 g given 20 min before induction | - Improved haemodynamic stability after laryngoscopy and intubation. - Lower requirement for postoperative opioid and later first analgesic request. - No significant difference in mean 1-min and 5-min Apgar scores of newborns. |
| Hassan, 2014 [12] | Saudi Arabia | Randomized, two-arm, prospective, unblinded trial | n = 58, pregnant women, ASA class I and II, scheduled for elective caesarean section, Age 18 to 39 years | General | - IV paracetamol 1 g given over 15–20 min, 30 min before induction - IV paracetamol 1 g given over 15–20 min, 30 min before the end of the operation | - Patients who received preoperative paracetamol had better hemodynamic stability, especially before delivery of the baby. - They also had lower requirements for intra- and postoperative opioids, longer duration of next analgesia needed and lower incidence of postoperative side effects. |
| Ozmete, 2016 [13] | Turkey | Randomized, placebo-controlled, double-blind trial | n = 60, pregnant women, ASA class I and II, scheduled for elective caesarean section, Age 18 to 40 years | General | - IV paracetamol 1 g given 15 min before induction | - Significantly reduced postoperative pain and opioid consumption within 24 h after caesarean section. - No significant difference in Apgar scores and patient side effects. |
| Prasanna, 2010 [14] | Oman | Randomized, two-arm, prospective, blinded trial | n = 80, pregnant women, ASA class I and II, scheduled for elective caesarean section, Mean Age 30.51 years | General | - IM diclofenac sodium 75 mg and IV paracetamol 1 g after induction, before surgical incision - IM diclofenac sodium 75 mg and IV paracetamol 1 g at the end of surgery | - Patients who received pre-incision analgesia had significantly fewer occurrences of incidental pain and reduced postoperative opioid requirements. |
| Soltani, 2015 [15] | Iran | Randomized, placebo-controlled, double-blind trial | n = 80, pregnant women, ASA class I and II, admitted for urgent caesarean section, Mean Age 28.49 ± 4.63 years | General | - IV paracetamol 15 mg/kg given 15 min before induction | - Significantly blunted heart rate changes following endotracheal intubation and reduced early postoperative pain. - Significantly lower requirements for intra- and postoperative opioids. |
| Towers, 2018 [16] | United States | Randomized, placebo-controlled, double-blind trial | n = 105, pregnant women, scheduled for elective caesarean section, Mean Age 27.1 ± 2.9 years | Spinal | - IV paracetamol 1 g given 15 min before surgical incision | - No difference in postoperative opioid requirements and length of stay postdelivery. - Administration of IV paracetamol did not result in elevated neonate cord blood paracetamol levels |
| Study (Author, Year) | Sequence Generation | Allocation Concealment | Blinding | Incomplete Outcome Data | Selective Outcome Reporting | Other Bias |
|---|---|---|---|---|---|---|
| Altenau, 2017 [10] |  |  |  |  |  |  |
| Ayatollahi, 2014 [11] |  |  |  |  |  |  |
| Hassan, 2014 [12] |  |  |  |  |  |  |
| Ozmete, 2016 [13] |  |  |  |  |  |  |
| Prasanna, 2010 [14] |  |  |  |  |  |  |
| Soltani, 2015 [15] |  |  |  |  |  |  |
| Towers, 2018 [16] |  |  |  |  |  |  |
 low risk of bias;
low risk of bias;  high risk of bias;
high risk of bias;  unclear risk of bias.
unclear risk of bias.| Q | 13.64 |
| DF | 4 |
| Significance level | P = 0.0086 |
| I2 (inconsistency) | 70.66% |
| 95% CI for I2 | 25.43 to 88.46 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, Q.X.; Loke, W.; Yeo, W.S.; Chng, K.Y.Y.; Tan, C.H. A Meta-Analysis of the Utility of Preoperative Intravenous Paracetamol for Post-Caesarean Analgesia. Medicina 2019, 55, 424. https://doi.org/10.3390/medicina55080424
Ng QX, Loke W, Yeo WS, Chng KYY, Tan CH. A Meta-Analysis of the Utility of Preoperative Intravenous Paracetamol for Post-Caesarean Analgesia. Medicina. 2019; 55(8):424. https://doi.org/10.3390/medicina55080424
Chicago/Turabian StyleNg, Qin Xiang, Wayren Loke, Wee Song Yeo, Kelvin Yong Yan Chng, and Chin How Tan. 2019. "A Meta-Analysis of the Utility of Preoperative Intravenous Paracetamol for Post-Caesarean Analgesia" Medicina 55, no. 8: 424. https://doi.org/10.3390/medicina55080424