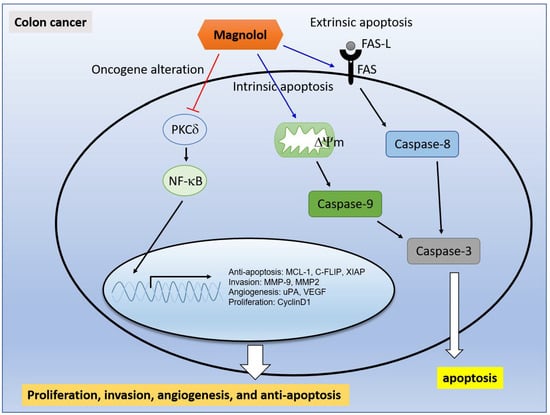
Suppression of PKCδ/NF-κB Signaling and Apoptosis Induction through Extrinsic/Intrinsic Pathways Are Associated with Magnolol-Inhibited Tumor Progression in Colorectal Cancer In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
2.1. Both Magnolol and PKC Inhibitor May Suppress NF-κB Signaling in CRC Cells
2.2. Magnolol Suppressed Tumor Cell Growth, PKC/NF-κB Signaling, Expression of NF-κB Mediated Downstream Proteins in CRC Cells
2.3. Magnolol Triggered Both Extrinsic and Intrinsic Apoptosis Effect in CRC Cells
2.4. Inhibition of PKCδ/NF-κB Signaling Was Associated with Magnolol-Diminished Invasion Ability of CRC Cells
2.5. Magnolol Effectively Suppressed CRC-Bearing Tumor Growth
2.6. Magnolol Suppressed PKCδ/NF-κB Signaling, NF-κB Related Downstream Proteins Expression and Promoted Apoptotic Aroteins Axpression
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Cell Viability (MTT Assay)
4.4. Transfection and Stable Clone Selection
4.5. Reporter Gene Assay
4.6. Caspase-3, -8, -9 Activity Analyses
4.7. Fas and Fas-L Analysis
4.8. Mitochondrial Membrane Potential (MMP) and Cellular Ca2+ Analysis
4.9. Invasion Tranwell Assay
4.10. Western Blot
4.11. Animal Experiment
4.12. Mice Computer Tomography (CT)
4.13. Hematoxylin and Eosin (H&E) Staining
4.14. Immunohistochemistry (IHC) Staining
4.15. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Kunzmann, A.T.; Coleman, H.G.; Huang, W.Y.; Kitahara, C.M.; Cantwell, M.M.; Berndt, S.I. Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am. J. Clin. Nutr. 2015, 102, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Oruc, Z.; Kaplan, M.A. Effect of exercise on colorectal cancer prevention and treatment. World J. Gastrointest. Oncol. 2019, 11, 348–366. [Google Scholar] [CrossRef] [PubMed]
- Piawah, S.; Venook, A.P. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer 2019, 125, 4139–4147. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, N.; Soltani, A.; Ghazvini, K.; Hassanian, S.M.; Hashemy, S.I. PD-1/PD-L1 blockade as a novel treatment for colorectal cancer. Biomed. Pharmacother. 2019, 110, 312–318. [Google Scholar] [CrossRef]
- Xu, Y.; Mao, J.J.; Sun, L.; Yang, L.; Li, J.; Hao, Y.; Li, H.; Hou, W.; Chu, Y.; Bai, Y.; et al. Association between Use of Traditional Chinese Medicine Herbal Therapy and Survival Outcomes in Patients with Stage II and III Colorectal Cancer: A Multicenter Prospective Cohort Study. J. Natl. Cancer Inst. 2017, 2017, 52. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Zhao, J.; Cong, W. Chinese Herbal Medicines Facilitate the Control of Chemotherapy-Induced Side Effects in Colorectal Cancer: Progress and Perspective. Front. Pharmacol. 2018, 9, 1442. [Google Scholar] [CrossRef]
- Chang, H.; Lei, L.; Zhou, Y.; Ye, F.; Zhao, G. Dietary Flavonoids and the Risk of Colorectal Cancer: An Updated Meta-Analysis of Epidemiological Studies. Nutrients 2018, 10, 950. [Google Scholar] [CrossRef] [Green Version]
- Cotterchio, M.; Boucher, B.A.; Manno, M.; Gallinger, S.; Okey, A.; Harper, P. Dietary phytoestrogen intake is associated with reduced colorectal cancer risk. Nutr. J. 2006, 136, 3046–3053. [Google Scholar] [CrossRef] [Green Version]
- Benarba, B.; Pandiella, A. Colorectal cancer and medicinal plants: Principle findings from recent studies. Biomed. Pharmacother. 2018, 107, 408–423. [Google Scholar] [CrossRef]
- Hsieh, S.L.; Hsieh, S.; Kuo, Y.H.; Wang, J.J.; Wang, J.C.; Wu, C.C. Effects of Panax notoginseng on the Metastasis of Human Colorectal Cancer Cells. Am. J. Chin. Med. 2016, 44, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Hong, J.T. Roles of NF-kappaB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef]
- Patel, M.; Horgan, P.G.; McMillan, D.C.; Edwards, J. NF-kappaB pathways in the development and progression of colorectal cancer. Transl. Res. 2018, 197, 43–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.C.; Chen, C.; Xu, Z.Q.; Zhao, J.K.; Ou, B.C.; Sun, J.; Zheng, M.H.; Zong, Y.P.; Lu, A.G. CCR6 promotes tumor angiogenesis via the AKT/NF-κB/VEGF pathway in colorectal cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Zhao, J.; Guan, S.; Feng, H.; Wangpu, X.; Zhu, C.; Zong, Y.; Ma, J.; Sun, J.; Shen, X.; et al. Correction: CCR4 promotes metastasis via ERK/NF-kappaB/MMP13 pathway and acts downstream of TNF-alpha in colorectal cancer. Oncotarget 2017, 8, 41779. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Evers, B.M. Induction of cIAP-2 in human colon cancer cells through PKC delta/NF-kappa B. J. Biol. Chem. 2003, 278, 51091–51099. [Google Scholar] [CrossRef] [Green Version]
- Choo, Z.; Loh, A.H.P.; Chen, Z.X. Destined to Die: Apoptosis and Pediatric Cancers. Cancers 2019, 11, 1623. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.M.; Yang, Z.J.; Xie, Q.; Zhang, Z.K.; Zhang, H.; Ma, J.Y. Natural products for treating colorectal cancer: A mechanistic review. Biomed. Pharmacother. 2019, 117, 109142. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kang, S.A.; Hong, M.S.; Park, H.J.; Kim, M.J.; Park, H.J.; Kim, H.K. Coptidis rhizoma induces apoptosis in human colorectal cancer cells SNU-C4. Am. J. Chin. Med. 2004, 32, 873–882. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Rane, G.; Kanchi, M.M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Tan, B.K.; Kumar, A.P.; Sethi, G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef]
- Zhu, H.; Hao, J.; Niu, Y.; Liu, D.; Chen, D.; Wu, X. Molecular targets of Chinese herbs: A clinical study of metastatic colorectal cancer based on network pharmacology. Sci. Rep. 2018, 8, 7238. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.P.; Lee, W.L.; Tang, Y.Q.; Yap, W.H. Honokiol: A Review of Its Anticancer Potential and Mechanisms. Cancers 2019, 12, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, H.; Chen, W.; Shen, L.; Sheng, Q.; Teng, L. Honokiol augments the anti-cancer effects of oxaliplatin in colon cancer cells. Acta Biochim. Biophys. Sin. 2013, 45, 773–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuan, L.Y.; Chen, W.L.; Chen, J.H.; Hsu, F.T.; Liu, T.T.; Chen, W.T.; Wang, K.L.; Chen, W.C.; Liu, Y.C.; Wang, W.S. Magnolol Induces Apoptosis and Inhibits ERK-modulated Metastatic Potential in Hepatocellular Carcinoma Cells. In Vivo 2018, 32, 1361–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.F.; Lee, T.S.; Lin, S.Y.; Hsu, S.P.; Juan, S.H.; Hsu, Y.H.; Zhong, W.B.; Lee, W.S. Involvement of Ras/Raf-1/ERK actions in the magnolol-induced upregulation of p21 and cell-cycle arrest in colon cancer cells. Mol. Carcinog. 2007, 46, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.L.; Hsieh, S.; Lai, P.Y.; Wang, J.J.; Li, C.C.; Wu, C.C. Carnosine Suppresses Human Colorectal Cell Migration and Intravasation by Regulating EMT and MMP Expression. Am. J. Chin. Med. 2019, 47, 477–494. [Google Scholar] [CrossRef]
- Bahnassy, A.A.; Zekri, A.-R.N.; El-Houssini, S.; El-Shehaby, A.M.R.; Mahmoud, M.R.; Abdallah, S.; El-Serafi, M. Cyclin A and cyclin D1 as significant prognostic markers in colorectal cancer patients. BMC Gastroenterol. 2004, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Pan, P.J.; Tsai, J.J.; Liu, Y.C. Amentoflavone Inhibits Metastatic Potential through Suppression of ERK/NF-kappaB Activation in Osteosarcoma U2OS Cells. Anticancer Res. 2017, 37, 4911–4918. [Google Scholar]
- Dong, W.; Li, H.; Zhang, Y.; Yang, H.; Guo, M.; Li, L.; Liu, T. Matrix metalloproteinase 2 promotes cell growth and invasion in colorectal cancer. Acta Biochim. Biophys. Sin. 2011, 43, 840–848. [Google Scholar] [CrossRef] [Green Version]
- Mackay, H.J.; Twelves, C.J. Protein kinase C: A target for anticancer drugs? Endocr. Relat. Cancer 2003, 10, 389–396. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Zhou, Y.; Evers, B.M. PKCdelta-mediated regulation of FLIP expression in human colon cancer cells. Int. J. Cancer 2006, 118, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunnumakkara, A.B.; Diagaradjane, P.; Guha, S.; Deorukhkar, A.; Shentu, S.; Aggarwal, B.B.; Krishnan, S. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin. Cancer Res. 2008, 14, 2128–2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, Y.C.; Lin, W.C.; Chiang, I.T.; Chang, Y.F.; Chen, C.W.; Su, S.H.; Chen, C.L.; Hwang, J.J. Sorafenib sensitizes human colorectal carcinoma to radiation via suppression of NF-kappaB expression in vitro and in vivo. Biomed. Pharmacother. 2012, 66, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Voboril, R.; Hochwald, S.N.; Li, J.; Brank, A.; Weberova, J.; Wessels, F.; Moldawer, L.L.; Camp, E.R.; MacKay, S.L. Inhibition of NF-kappa B augments sensitivity to 5-fluorouracil/folinic acid in colon cancer. J. Surg. Res. 2004, 120, 178–188. [Google Scholar] [CrossRef]
- Fang, J.Y.; Richardson, B.C. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005, 6, 322–327. [Google Scholar] [CrossRef]
- Tai, C.J.; Chang, C.C.; Jiang, M.C.; Yeh, C.M.; Su, T.C.; Wu, P.R.; Chen, C.J.; Yeh, K.T.; Lin, S.H.; Chen, H.C. Clinical-pathological correlation of K-Ras mutation and ERK phosphorylation in colorectal cancer. Pol. J. Pathol. 2012, 63, 93–100. [Google Scholar]
- Malinowsky, K.; Nitsche, U.; Janssen, K.P.; Bader, F.G.; Spath, C.; Drecoll, E.; Keller, G.; Hofler, H.; Slotta-Huspenina, J.; Becker, K.F. Activation of the PI3K/AKT pathway correlates with prognosis in stage II colon cancer. Br. J. Cancer 2014, 110, 2081–2089. [Google Scholar] [CrossRef] [Green Version]
- Noble, P.; Vyas, M.; Al-Attar, A.; Durrant, S.; Scholefield, J.; Durrant, L. High levels of cleaved caspase-3 in colorectal tumour stroma predict good survival. Br. J. Cancer 2013, 108, 2097–2105. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.G.; Wang, C.; Li, Y.; Wang, L.; Zhou, B.; Xu, B.; Jiang, X.; Zhou, Z.G.; Sun, X.F. Downregulation of caspase-9 is a frequent event in patients with stage II colorectal cancer and correlates with poor clinical outcome. Color. Dis. 2010, 12, 1213–1218. [Google Scholar] [CrossRef]
- Kykalos, S.; Mathaiou, S.; Karayiannakis, A.J.; Patsouras, D.; Lambropoulou, M.; Simopoulos, C. Tissue expression of the proteins fas and fas ligand in colorectal cancer and liver metastases. J. Gastrointest. 2012, 43, 224–228. [Google Scholar] [CrossRef]
- Plati, J.; Bucur, O.; Khosravi-Far, R. Dysregulation of apoptotic signaling in cancer: Molecular mechanisms and therapeutic opportunities. J. Cell. Biochem. 2008, 104, 1124–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, J.; Wang, P.; Tan, S.; Chen, D.; Nikolovska-Coleska, Z.; Zou, F.; Yu, J.; Zhang, L. Mcl-1 Degradation Is Required for Targeted Therapeutics to Eradicate Colon Cancer Cells. Cancer Res. 2017, 77, 2512–2521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longley, D.B.; Wilson, T.R.; McEwan, M.; Allen, W.L.; McDermott, U.; Galligan, L.; Johnston, P.G. c-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene 2006, 25, 838–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flanagan, L.; Kehoe, J.; Fay, J.; Bacon, O.; Lindner, A.U.; Kay, E.W.; Deasy, J.; McNamara, D.A.; Prehn, J.H. High levels of X-linked Inhibitor-of-Apoptosis Protein (XIAP) are indicative of radio chemotherapy resistance in rectal cancer. Radiat. Oncol. 2015, 10, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.Y.; Chang, Y.T.; Liu, J.D.; Yu, C.H.; Ho, Y.S.; Lee, Y.H.; Lee, W.S. Molecular mechanisms of apoptosis induced by magnolol in colon and liver cancer cells. Mol. Carcinog. 2001, 32, 73–83. [Google Scholar] [CrossRef]
- Weng, M.C.; Li, M.H.; Chung, J.G.; Liu, Y.C.; Wu, J.Y.; Hsu, F.T.; Wang, H.E. Apoptosis induction and AKT/NF-kappaB inactivation are associated with regroafenib-inhibited tumor progression in non-small cell lung cancer in vitro and in vivo. Biomed. Pharmacother. 2019, 116, 109032. [Google Scholar] [CrossRef]
- Chen, W.T.; Chen, Y.K.; Lin, S.S.; Hsu, F.T. Hyperforin Suppresses Tumor Growth and NF-kappaB-mediated Anti-apoptotic and Invasive Potential of Non-small Cell Lung Cancer. Anticancer Res. 2018, 38, 2161–2167. [Google Scholar]
- Chiang, I.T.; Liu, Y.C.; Wang, W.H.; Hsu, F.T.; Chen, H.W.; Lin, W.J.; Chang, W.Y.; Hwang, J.J. Sorafenib inhibits TPA-induced MMP-9 and VEGF expression via suppression of ERK/NF-kappaB pathway in hepatocellular carcinoma cells. In Vivo 2012, 26, 671–681. [Google Scholar]
- Pan, P.J.; Liu, Y.C.; Hsu, F.T. Protein Kinase B and Extracellular Signal-Regulated Kinase Inactivation is Associated with Regorafenib-Induced Inhibition of Osteosarcoma Progression In Vitro and In Vivo. J. Clin. Med. 2019, 8, 900. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.H.; Chung, J.G.; Hsu, F.T. Regorefenib induces extrinsic/intrinsic apoptosis and inhibits MAPK/NF-kappaB-modulated tumor progression in bladder cancer in vitro and in vivo. Environ. Toxicol. 2019, 34, 679–688. [Google Scholar] [CrossRef]
- Cardiff, R.D.; Miller, C.H.; Munn, R.J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb. Protoc. 2014, 2014, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.T.; Chiang, I.T.; Kuo, Y.C.; Hsia, T.C.; Lin, C.C.; Liu, Y.C.; Chung, J.G. Amentoflavone Effectively Blocked the Tumor Progression of Glioblastoma via Suppression of ERK/NF-kappa B Signaling Pathway. Am. J. Chin. Med. 2019, 47, 913–931. [Google Scholar] [CrossRef] [PubMed]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.-M.; Weng, Y.-S.; Kuan, L.-Y.; Chen, J.-H.; Hsu, F.-T. Suppression of PKCδ/NF-κB Signaling and Apoptosis Induction through Extrinsic/Intrinsic Pathways Are Associated with Magnolol-Inhibited Tumor Progression in Colorectal Cancer In Vitro and In Vivo. Int. J. Mol. Sci. 2020, 21, 3527. https://doi.org/10.3390/ijms21103527
Su C-M, Weng Y-S, Kuan L-Y, Chen J-H, Hsu F-T. Suppression of PKCδ/NF-κB Signaling and Apoptosis Induction through Extrinsic/Intrinsic Pathways Are Associated with Magnolol-Inhibited Tumor Progression in Colorectal Cancer In Vitro and In Vivo. International Journal of Molecular Sciences. 2020; 21(10):3527. https://doi.org/10.3390/ijms21103527
Chicago/Turabian StyleSu, Chun-Min, Yueh-Shan Weng, Lin-Yen Kuan, Jiann-Hwa Chen, and Fei-Ting Hsu. 2020. "Suppression of PKCδ/NF-κB Signaling and Apoptosis Induction through Extrinsic/Intrinsic Pathways Are Associated with Magnolol-Inhibited Tumor Progression in Colorectal Cancer In Vitro and In Vivo" International Journal of Molecular Sciences 21, no. 10: 3527. https://doi.org/10.3390/ijms21103527