Effects of Flutriafol Fungicide on the Lipid Accumulation in Human Liver Cells and Rat Liver
Abstract
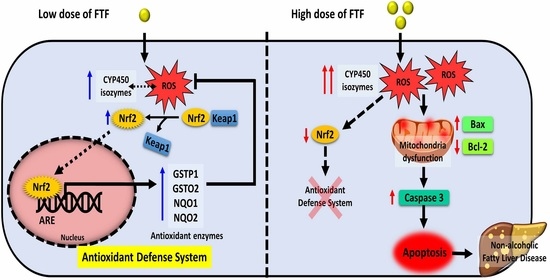
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture and Treatment
2.3. Lactate Dehydrogenase (LDH) Activity Assay
2.4. Cell Viability Assay
2.5. Oil Red O Staining
2.6. Measurement of Cellular Oxidative Stress
2.7. Measurement of Mitochondrial Membrane Potential
2.8. Determination of the Gene Expression Levels of Cytochrome P450 and Antioxidant Enzymes
2.9. Determination of Nuclear Translocation of Nrf2 Using Nuclear Fractionation and Immunofluorescence Microscopy
2.10. Determination of Apoptosis Using Annexin V/Propidium Iodide Assay
2.11. Animal Experiments
2.12. Biochemical Analysis of Blood Plasma
2.13. Histological Analysis
2.14. Determination of Protein Expression Level of Nrf2 and Apoptosis-Related Markers Using Western Blot Analysis
2.15. Statistical Analysis
3. Results
3.1. Flutriafol-Induced Cell Damage, Reduced Cell Viability, and Lipid Accumulation in Human Liver Cells
3.2. Flutriafol-Induced Cytochrome P450 Activation in Human Liver Cells
3.3. Flutriafol-Induced Oxidative Stress and Mitochondria Membrane Potential in Human Liver Cells
3.4. Flutriafol-Induced Intracellular Signaling Pathway and Antioxidant Enzymes in Human Liver Cells
3.5. Flutriafol-Induced Apoptosis in Human Liver Cells
3.6. Flutriafol-Induced Changes in Body Weight, Feed Intake, Organ Weight/Body Weight Ratio, and Biochemical Profiles in Rats
3.7. Flutriafol-Induced Activation of Cytochrome P450 and Nrf2 Signaling in Rat Liver
3.8. Flutriafol-Induced Apoptosis in Rat Liver
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gianessi, L.; Reigner, N. The importance of fungicides in US crop production. Outlooks Pest Manag. 2006, 17, 209. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Van den Bosch, F.; Oliver, R.; Heick, T.; Paveley, N. Targeting fungicide inputs according to need. Annu. Rev. Phytopathol. 2017, 55, 181–203. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.-C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Li, Y.; Dong, F.; Liu, X.; Xu, J.; Li, J.; Kong, Z.; Chen, X.; Liang, X.; Zheng, Y. Simultaneous enantioselective determination of triazole fungicides in soil and water by chiral liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2012, 1224, 51–60. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for flutriafol according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2014, 12, 3687. [Google Scholar] [CrossRef]
- Joint FAO/WHO Meeting on Pesticide Residues (JMPR). Pesticide Residues in Food-2005: Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Resideues, Rome, Italy, 20–29 September 2005; World Helath Organization: Geneva, Switzerland, 2006; Food and Agriculture Organization of the United Nations: Rome, Italy, 2005; Available online: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Report11/Flutriafol.pdf (accessed on 14 May 2021).
- Zarn, J.A.; Brüschweiler, B.J.; Schlatter, J.R. Azole fungicides affect mammalian steroidogenesis by inhibiting sterol 14 alpha-demethylase and aromatase. Environ. Health Perspect. 2003, 111, 255–261. [Google Scholar] [CrossRef]
- Ziogas, B.N.; Malandrakis, A.A. Sterol biosynthesis inhibitors: C14 demethylation (DMIs). In Fungicide Resistance in Plant Pathogens; Springer: Berlin/Heidelberg, Germany, 2015; pp. 199–216. [Google Scholar]
- Joint FAO/WHO Meeting on Pesticide Residues (JMPR). Pesticide Residues in Food-2011: Toxicological Evaluations/Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Geneva, Switzerland, 20–29 September 2011; World Health Organization: Geneva, Switzerland, 2012; Available online: https://apps.who.int/iris/bitstream/handle/10665/75147/9789241665278_eng.pdf?sequence=1&isAllowed=y (accessed on 14 May 2021).
- Xu, C.; Li, C.Y.-T.; Kong, A.-N.T. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 2005, 28, 249–268. [Google Scholar] [CrossRef]
- Abass, K.; Turpeinen, M.; Rautio, A.; Hakkola, J.; Pelkonen, O. Metabolism of pesticides by human cytochrome P450 enzymes in vitro—A survey. In Insecticides: Advances in Integrated Pest Management; InTech: Rijeka, Croatia, 2012; Volume 1, pp. 165–194. [Google Scholar]
- Almazroo, O.A.; Miah, M.K.; Venkataramanan, R. Drug metabolism in the liver. Clin. Liver Dis. 2017, 21, 1–20. [Google Scholar] [CrossRef]
- Veith, A.; Moorthy, B. Role of cytochrome P450s in the generation and metabolism of reactive oxygen species. Curr. Opin. Toxicol. 2018, 7, 44–51. [Google Scholar] [CrossRef]
- Ekstedt, M.; Nasr, P.; Kechagias, S. Natural history of NAFLD/NASH. Curr. Hepatol. Rep. 2017, 16, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Mundi, M.S.; Velapati, S.; Patel, J.; Kellogg, T.A.; Abu Dayyeh, B.K.; Hurt, R.T. Evolution of NAFLD and its management. Nutr. Clin. Pract. 2020, 35, 72–84. [Google Scholar] [CrossRef]
- Sumida, Y.; Yoneda, M. Current and future pharmacological therapies for NAFLD/NASH. J. Gastroenterol. 2018, 53, 362–376. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.S.; Park, Y. Insecticide exposure and development of nonalcoholic fatty liver disease. J. Agric. Food Chem. 2018, 66, 10132–10138. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Guo, G.L. Understanding environmental contaminants’ direct effects on non-alcoholic fatty liver disease progression. Curr. Environ. Health Rep. 2019, 6, 95–104. [Google Scholar] [CrossRef]
- Stellavato, A.; Lamberti, M.; Pirozzi, A.V.A.; de Novellis, F.; Schiraldi, C. Myclobutanil worsens nonalcoholic fatty liver disease: An in vitro study of toxicity and apoptosis on HepG2 cells. Toxicol. Lett. 2016, 262, 100–104. [Google Scholar] [CrossRef]
- Damba, T.; Bourgonje, A.R.; Abdulle, A.E.; Pasch, A.; Sydor, S.; van den Berg, E.H.; Gansevoort, R.T.; Bakker, S.J.; Blokzijl, H.; Dullaart, R.P. Oxidative stress is associated with suspected non-alcoholic fatty liver disease and all-cause mortality in the general population. Liver Int. 2020, 40, 2148–2159. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Carter-Kent, C.; Feldstein, A.E. Apoptosis in nonalcoholic fatty liver disease: Diagnostic and therapeutic implications. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 201–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, A.; Huerta-Salgado, C.; Orozco-Aguilar, J.; Aguirre, F.; Tacchi, F.; Simon, F.; Cabello-Verrugio, C. Role of Oxidative Stress in Hepatic and Extrahepatic Dysfunctions during Nonalcoholic Fatty Liver Disease (NAFLD). Oxidative Med. Cell. Longev. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Rahman, M.S. Pesticide Residue in Foods: Sources, Management, and Control; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Medina-Pastor, P.; Triacchini, G. The 2018 European Union report on pesticide residues in food. EFSA J. 2020, 18, e06057. [Google Scholar]
- European Food Safety Authority. Reasoned opinion on the modification of the existing MRLs for flutriafol in pome fruits, peaches, cherries and plums. EFSA J. 2013, 11, 3446. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority. Modification of the existing MRLs for flutriafol in various crops. EFSA J. 2010, 8, 1587. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, K.; Mukai, M.; Ramaiah, S.K. Liver Toxicity. In Veterinary Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 239–257. [Google Scholar]
- Manikandan, P.; Nagini, S. Cytochrome P450 structure, function and clinical significance: A review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.-O.; Steinbrenner, H. Cellular adaptation to xenobiotics: Interplay between xenosensors, reactive oxygen species and FOXO transcription factors. Redox Biol. 2017, 13, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, M.; Hiraishi, A.; Touyama, M.; Sakamoto, K. Oxidative stress induced lipid accumulation via SREBP1c activation in HepG2 cells. Biochem. Biophys. Res. Commun. 2008, 375, 602–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubbins, P.O. Triazole antifungal agents drug–drug interactions involving hepatic cytochrome P450. Expert Opin. Drug Metab. Toxicol. 2011, 7, 1411–1429. [Google Scholar] [CrossRef]
- Knebel, C.; Heise, T.; Zanger, U.M.; Lampen, A.; Marx-Stoelting, P.; Braeuning, A. The azole fungicide tebuconazole affects human CYP1A1 and CYP1A2 expression by an aryl hydrocarbon receptor-dependent pathway. Food Chem. Toxicol. 2019, 123, 481–491. [Google Scholar] [CrossRef]
- Zhuang, S.L.; Bao, L.L.; Wang, H.F.; Zhang, M.; Yang, C.; Zhou, X.Y.; Wu, Y.; Rehman, K.; Naranmandura, H. The involvement of ER-stress and ROS generation in difenoconazole-induced hepatocellular toxicity. Toxicol. Res. 2015, 4, 1195–1203. [Google Scholar] [CrossRef]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; Von Knethen, A. Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Jiang, Y.-F.; Ponnusamy, M.; Diallo, M. Role of Nrf2 in chronic liver disease. World J. Gastroenterol. 2014, 20, 13079. [Google Scholar] [CrossRef]
- Lee, J.-S.; Surh, Y.-J. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005, 224, 171–184. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.-F.; Chen, P.-C.; Lin, Y.-C.; Chou, K.-Y.; Chen, H.-E.; Ho, C.-Y.; Lin, J.-F.; Hwang, T.I.-S. Miconazole contributes to NRF2 activation by noncanonical P62-KEAP1 pathway in bladder cancer cells. Drug Des. Dev. Ther. 2020, 14, 1209. [Google Scholar] [CrossRef] [Green Version]
- Haegler, P.; Joerin, L.; Krähenbühl, S.; Bouitbir, J. Hepatocellular toxicity of imidazole and triazole antimycotic agents. Toxicol. Sci. 2017, 157, 183–195. [Google Scholar] [CrossRef] [Green Version]
- de la Vega, M.R.; Chapman, E.; Zhang, D.D. NRF2 and the hallmarks of cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef]
- Cao, L.; Quan, X.-B.; Zeng, W.-J.; Yang, X.-O.; Wang, M.-J. Mechanism of hepatocyte apoptosis. J. Cell Death 2016, 9, JCD-S39824. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Othmène, Y.B.; Hamdi, H.; Salem, I.B.; Annabi, E.; Amara, I.; Neffati, F.; Najjar, M.F.; Abid-Essefi, S. Oxidative stress, DNA damage and apoptosis induced by tebuconazole in the kidney of male Wistar rat. Chem. Biol. Interact. 2020, 330, 109114. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Peyton, L.; Gallagher, S.; Hashemzadeh, M. Triazole antifungals: A review. Drugs Today 2015, 51, 705–718. [Google Scholar] [CrossRef]
- Hall, A.; Elcombe, C.; Foster, J.; Harada, T.; Kaufmann, W.; Knippel, A.; Küttler, K.; Malarkey, D.; Maronpot, R.; Nishikawa, A. Liver hypertrophy: A review of adaptive (adverse and non-adverse) changes—Conclusions from the 3rd International ESTP Expert Workshop. Toxicol. Pathol. 2012, 40, 971–994. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-H.; Namgung, J.-S.; Lee, J.; Moon, D.-H.; Lee, H.-K. Analysis of biochemical markers related to Fatty liver patients. J. Phys. Ther. Sci. 2014, 26, 1865–1868. [Google Scholar] [CrossRef] [Green Version]
- Francoz, C.; Nadim, M.K.; Durand, F. Kidney biomarkers in cirrhosis. J. Hepatol. 2016, 65, 809–824. [Google Scholar] [CrossRef] [Green Version]
- De Chiara, F.; Heebøll, S.; Marrone, G.; Montoliu, C.; Hamilton-Dutoit, S.; Ferrandez, A.; Andreola, F.; Rombouts, K.; Grønbæk, H.; Felipo, V. Urea cycle dysregulation in non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 905–915. [Google Scholar] [CrossRef] [Green Version]
- Cobbina, E.; Akhlaghi, F. Non-alcoholic fatty liver disease (NAFLD)–pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metabol. Rev. 2017, 49, 197–211. [Google Scholar] [CrossRef]
- Sun, J.; Lu, Y.; Li, Y.; Pan, J.; Liu, C.; Gong, Z.; Huang, J.; Zheng, J.; Zheng, L.; Li, Y. Influence of Shenxiong glucose injection on the activities of six CYP isozymes and metabolism of warfarin in rats assessed using probe cocktail and pharmacokinetic approaches. Molecules 2017, 22, 1994. [Google Scholar] [CrossRef] [Green Version]
- Spahis, S.; Delvin, E.; Borys, J.-M.; Levy, E. Oxidative stress as a critical factor in nonalcoholic fatty liver disease pathogenesis. Antioxid. Redox Signal. 2017, 26, 519–541. [Google Scholar] [CrossRef]
- Wang, B.; Li, L.; Fu, J.; Yu, P.; Gong, D.; Zeng, C.; Zeng, Z. Effects of long-chain and medium-chain fatty acids on apoptosis and oxidative stress in human liver cells with steatosis. J. Food Sci. 2016, 81, H794–H800. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Canbay, A.; Angulo, P.; Taniai, M.; Burgart, L.J.; Lindor, K.D.; Gores, G.J. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 2003, 125, 437–443. [Google Scholar] [CrossRef]
- Kanda, T.; Matsuoka, S.; Yamazaki, M.; Shibata, T.; Nirei, K.; Takahashi, H.; Kaneko, T.; Fujisawa, M.; Higuchi, T.; Nakamura, H. Apoptosis and non-alcoholic fatty liver diseases. World J. Gastroenterol. 2018, 24, 2661. [Google Scholar] [CrossRef]








| Gene 1 | Primer Sequence 5′‒3′ |
|---|---|
| CYP1A2 (Human) | (F) ATG GCA TTG TCC CAG TCT G (R) TCT GGT GGA CTT TTC AGG C |
| CYP2C9 (Human) | (F) ATG GAT TCT CTT GTG GTC CTT (R) CAA TCA CTG GGA GAG GAG TG |
| CYP2C19 (Human) | (F) ATG GAT CCT TTT GTG GTC C (R) TAG GAT ATT TCC AAT CAC TGG G |
| CYP3A4 (Human) | (F) ATG GCT CTC ATC CCA GAC TTG G (R) CCC TGG AAT TCC AAG CTT CTT |
| CYP51A1 (Human) | (F) ACC TCT TGT CCA TGC TGC TGA T (R) TGG CAT GCC CAA GGA ATG GA |
| CYP2E1 (Human) | (F) TGC TGG TGT CCA TGT GGA G (R) CGG GTG AAG GAC TTG GGA AT |
| NQO1 (Human) | (F) GAC CTC TAT GCC ATG AAC TT (R) TAT AAG CCA GAA CAG ACT CG |
| NQO2 (Human) | (F) GAG TGG AAA CCC ACG AAG (R) AGC AAA CCG GAA TCG TAG |
| GSTP1 (Human) | (F) CAG ATC AGG GCC AGA GCT GGA A (R) GGT GAC GCA GGA TGG TAT TGG ACT |
| GSTO2 (Human) | (F) CTC CTA CTC TCG GGC TTC CAA A (R) AGA AAC AGC TGC GC CTG G |
| GAPDH (Human) | (F) GAC CCC TTC ATT GAC CTC AAC TAC (R)ATG ACA AGC TTC CCG TTC TCA G |
| CYP1A2 (Rat) | (F) AAA CCA GTG GCA GGT CAA CCA T (R) TCA CCT TCT CAC TCA GGG TCT TGT |
| CYP3A2 (Rat) | (F) ACG TTC ACC AGT GGA AGA CTC AAG (R) ACA TCC ATG CTG TAG GCA CCA A |
| GAPDH (Rat) | (F) TGC CAT CAA CGA CCC CTT CAT T (R) GCA TCA CCC CAT TTG ATG TTA GCG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.-C.; Sohn, H.; Kim, D.-H.; Jeong, C.-H.; Kim, D.-W.; Han, S.-G. Effects of Flutriafol Fungicide on the Lipid Accumulation in Human Liver Cells and Rat Liver. Foods 2021, 10, 1346. https://doi.org/10.3390/foods10061346
Kwon H-C, Sohn H, Kim D-H, Jeong C-H, Kim D-W, Han S-G. Effects of Flutriafol Fungicide on the Lipid Accumulation in Human Liver Cells and Rat Liver. Foods. 2021; 10(6):1346. https://doi.org/10.3390/foods10061346
Chicago/Turabian StyleKwon, Hyuk-Cheol, Hyejin Sohn, Do-Hyun Kim, Chang-Hee Jeong, Dong-Wook Kim, and Sung-Gu Han. 2021. "Effects of Flutriafol Fungicide on the Lipid Accumulation in Human Liver Cells and Rat Liver" Foods 10, no. 6: 1346. https://doi.org/10.3390/foods10061346