Immunotherapy in treatment naïve advanced non-small cell lung cancer
Introduction
Lung cancer is the leading cause of mortality and the second most common malignancy in both genders in the United States (1). Approximately 57% of patients with non-small cell lung cancer (NSCLC) present with stage IV disease at initial diagnosis (1). Platinum-based chemotherapy has for long been the mainstay first-line therapy for patients with metastatic NSCLC (2-5). However, chemotherapy renders a response rate (RR) of 15% to 33%, progression-free survival (PFS) of 3.1 to 5.5 months, and overall survival (OS) of less than 1 year (6-8). Multiple targeted and cytotoxic agents have been added to platinum-doublet chemotherapy, but only bevacizumab and necitumumab have shown modest improvements in PFS and OS in patients with non-squamous and squamous histology respectively (5,9,10). Significant progress in the last 10 years has originated from the identification of specific genetic changes that characterize subtypes of NSCLC (e.g., EGFR, ALK, ROS-1), and oral tyrosine kinase inhibitors (TKIs) became the standard front-line therapy for advanced non-squamous NSCLC harboring these mutations. The RR and PFS associated with frontline TKIs are approximately 56% to 80%, and 9.7–13.6 months, respectively. However, targeted therapy has failed to demonstrate an OS benefit when compared to chemotherapy (11-16). More recently, nevertheless, immunotherapy has dramatically changed the landscape of NSCLC treatment, and have clearly defined a new treatment era for patients with lung cancer.
The immune system has a vital role in regulating tumor growth, and the aim of checkpoint inhibition is to enhance and restore the ability of the immune system to recognize and eliminate tumor cells by overcoming the mechanisms used by malignancies to evade the immune response. The antibodies that target cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and programmed cell death protein-1 (PD-1) immune checkpoint pathways work by reestablishing the loss of immune responses towards cancer cells (17). Briefly, CD28 is a costimulatory molecule expressed on T cells that attaches to B7-1 (CD80) and B7-2 (CD86) ligands on antigen presenting cells (APCs), resulting in further stimulation of T cells. CTLA-4 is an inhibitory protein expressed on T cells that also binds to the B7 ligands; however, it interacts with B7 with much stronger affinity, overcoming CD28 stimulatory signal. Antibodies targeting CTLA-4 interrupt the interaction between CTLA-4 and B7 molecules and thus prevent T cell inactivation. Similarly to CTLA-4, PD-1 is also expressed on T cells and the connection between PD-1 and its ligands, programmed death-ligand 1 (PD-L1) and programmed death-ligand 2 (PD-L2), on APCs surface leads to T cell inactivation (18,19). Inhibition of this interaction by antibodies targeting the PD-1 pathway, leads to a reactivation of the cytotoxic T-cells resulting in tumor cell.
Nivolumab and pembrolizumab are both IgG4 monoclonal antibodies that attach to PD-1 receptors expressed on T cells and disrupt the binding to PD-L1, thereby restoring the cytotoxic T-cell effector function (20). Atezolizumab, on the other hand, is an IgG1 monoclonal anti-PD-L1 antibody that blocks the interaction between PD-L1/PD-1, and restores T-cell activity (21). Over the last 2 years, nivolumab, pembrolizumab and atezolizumab have been approved as a second-line therapy for metastatic NSCLC after platinum-based chemotherapy (22-25). Success with the checkpoint inhibitors in the second-line setting led to their evaluation in the first-line. More recently, the Food and Drug Administration (FDA) approved pembrolizumab as a single agent for first-line therapy for advanced NSCLC whose tumors express ≥50% PD-L1, and in combination with carboplatin plus pemetrexed for advanced non-squamous histology with any PD-L1 expression. This review will discuss the recent advances of immunotherapy in the frontline management of NSCLC.
Single agent PD-1 antibodies
Nivolumab
Given the efficacy of nivolumab in the second-line, CheckMate-012 trial tested single agent nivolumab in the first-line setting. This was a phase 1, multi-cohort study that enrolled 52 patients with stage IIIB to IV to receive nivolumab 3 mg/kg every 2 weeks until disease progression or unacceptable toxicity. The therapy was overall well tolerated and treatment-related adverse events (AEs) were reported in 71% of the patients. The most common side effects were consistent with previous nivolumab studies and included fatigue, nausea, diarrhea, pruritus, and arthralgia. Grade 3–4 AEs occurred in 10 patients (19%), and grade 3 rash was the most common event. The confirmed overall response rate (ORR) was 23%. ORR was 28% in patients with any PD-L1 expression (≥1%), and 14% in those with absence of PD-L1 expression. Responses were observed independently of PD-L1 expression; however, there was a tendency towards improved response as the PD-L1 levels increased. The median OS was 19.4 months, and there was no evident correlation between PFS/OS and PD-L1 expression (26).
Nivolumab monotherapy demonstrated promising RR in the first-line setting, however it did not translate into an advantage over chemotherapy in a large phase 3 trial. CheckMate-026 was a phase 3 randomized study of 541 patients that evaluated nivolumab monotherapy 3 mg/kg IV every 2 weeks versus platinum-based doublet chemotherapy every 3 weeks for stage IV NSCLC with PD-L1 tumor expression ≥1%. The primary endpoint of the study was PFS in patients with PD-L1 expression ≥5%. The median PFS was 4.2 months in the nivolumab arm and 5.9 months in the chemotherapy arm (HR, 1.15; 95% CI, 0.91 to 1.45; P=0.25). The median OS was also not different between the arms, 14.4 months with nivolumab and 13.2 months with chemotherapy (HR, 1.02; 95% CI, 0.80 to 1.30) (27). Nivolumab failed to meet the primary endpoint of superior PFS in patients with PD-L1 ≥5%, compared to chemotherapy. A plausible explanation for the lack of PFS benefit could be the low PD-L1 expression threshold used in the CheckMate-026 for patient selection. However, a subgroup analysis also failed to demonstrate a PFS and OS benefit in the PD-L1 ≥50% expression cohort (in contrast to KEYNOTE-024, discussed below). Evidently, PD-L1 expression is not an ideal marker to predict response to immunotherapy. Interestingly, an exploratory analysis of the Checkmate-026 revealed that high-tumor mutation burden (TMB) was associated with higher RR and PFS in the nivolumab group, compared to the chemotherapy group (28). Perhaps, the application of adjunct tools such as gene signatures, mutational load, and other biomarkers could better elucidate which patients will respond to checkpoint inhibition (29,30).
The CheckMate-227 study is currently accruing patients, irrespective of PD-L1 expression, and it randomizes 1:1:1 to nivolumab, or nivolumab and ipilimumab, or nivolumab and chemotherapy (NCT02477826). This trial will allow for further exploration and understanding of the usage of upfront nivolumab in a broad group of patients whose tumors have different PD-L1 expression levels. CheckMate-370 trial is an ongoing phase 1/2 study using nivolumab as maintenance after induction chemotherapy or as first-line treatment alone or in combination with multiple standard first-line chemotherapy options (NCT02574078).
Pembrolizumab
KEYNOTE-024 was a phase 3 study that randomized 305 patients with untreated metastatic NSCLC with PD-L1 expression level ≥50% and no targetable drivers (EGFR or ALK) to receive either pembrolizumab 200 mg every 3 weeks or platinum-based chemotherapy, which included the use of pemetrexed maintenance for patients with non-squamous histology. The primary endpoint was PFS and secondary endpoints were RR, OS, and safety. Approximately 30% of the samples had a PD-L1 expression of ≥50%. The median PFS was 10.3 months (95% CI, 6.7 to not reached) in the immunotherapy arm compared to 6.0 months (95% CI, 4.2 to 6.2) in the chemotherapy arm [hazard ratio (HR) for disease progression or death, 0.50; 95% CI, 0.37 to 0.68; P<0.001]. Six-month OS was 80.2% in the pembrolizumab arm versus 72.4% in the chemotherapy arm (HR, 0.6; 95% CI, 0.41–0.89; P=0.005), with median OS not reached in either group. The RR was also superior in the pembrolizumab group compared to the chemotherapy group (44.8% vs. 27.8%). Pembrolizumab had fewer treatment-related AEs compared to chemotherapy (73.4% vs. 90.0%), and less grade 3–5 AEs (26.6% vs. 53.3%). The trial was stopped early by the Data and Safety Monitoring Committee (DSMC) and the patients in the chemotherapy group were offered pembrolizumab (31). Based on these results, the FDA approved in October of 2016 the use of first-line pembrolizumab for patients with advanced NSCLC, PD-L1 expression ≥50% and no driver mutations, regardless of histology.
In a recent update, the progression-free survival in the second line (PFS2) and updated OS results were presented. PFS2 was defined as time from randomization to objective tumor progression on next-line therapy or death from any cause, whichever occurred first. After 19.1 months of follow-up, second-line therapy was received by 31.2% of patients in the pembrolizumab group, and 64.2% in the chemotherapy group. The median PFS2 was 18.3 months in the pembrolizumab arm [95% CI, 12.7–not estimable (NE)], versus 8.4 months in the chemotherapy arm (95% CI, 6.8–9.8). The median OS had not been reached yet in the pembrolizumab group (95% CI, 19.4–NE), compared to 14.5 months in the chemotherapy group (95% CI, 9.8–19.6). Thus, pembrolizumab continued to show OS benefit over chemotherapy in the front-line setting for advanced NSCLC patients and PD-L1 expression ≥50%. These results suggest that in patients with high PD-L1 expression, first line immunotherapy followed by chemotherapy was more likely to be beneficial than the reverse sequence.
The KEYNOTE-024 was a landmark clinical trial, which has established pembrolizumab as the new first-line therapy for patients with NSCLC and PD-L1 expression ≥50%. As of today, immunohistochemistry for PD-L1 expression should be tested in every patient with newly diagnosed advanced NSCLC, irrespectively of histology. Of note, KEYNOTE-042 is an ongoing phase 3 trial comparing pembrolizumab to platinum-based chemotherapy in newly diagnosed patients with any level of PD-L1 expression (NCT02220894).
Single agent PD-L1 antibodies
Atezolizumab
Atezolizumab is currently approved for the treatment of metastatic NSCLC whose disease progressed during or following platinum-based chemotherapy (21,24). The BIRCH study was a phase 2 single-arm trial designed to assess the efficacy of atezolizumab in advanced NSCLC across different lines of therapy. The trial included three cohorts: cohort 1 (no prior chemotherapy); cohort 2 (progression after one platinum-based regimen); and cohort 3 (progression after at least two prior lines of chemotherapy). Only patients with PD-L1 expression on ≥5% of tumor cells (TC; TC2/3) or tumor-infiltrating immune cells (ICs; IC2/3) on the SP142 immunohistochemistry assay were enrolled. PD-L1 TC expression was scored as a percentage of PD-L1—positive TC (TC3 ≥50% or TC2 ≥5% but <50%). PD-L1 IC expression was scored as a percentage of tumor area stained positive (IC3 ≥10% or IC2 ≥5% but, <10%). For all cohorts, atezolizumab 1,200 mg was administered IV every 3 weeks. The primary endpoint was ORR, and secondary endpoints were median duration of response, PFS, and OS. Of the 659 patients who received atezolizumab across all cohorts, only 139 patients were treatment naïve (cohort 1). The ORR was 22%, 19% and 18% for cohorts 1, 2, and 3, respectively. RR in the TC3 or IC3 group, was 31%, 26%, and 27% for cohorts 1, 2, and 3, respectively. Among responders, the median duration of response was 9.8 months, NE, and 11.8 months for cohorts 1, 2, and 3, respectively. For the TC3 or IC3 group, median duration of response was 10.0 months, NE, and 7.2 months for cohorts 1, 2, and 3, respectively. The median PFS was 5.4 months for cohort 1 (95% CI, 3.0 to 6.9 months), 2.8 months for cohort 2 (95% CI, 1.5 to 3.9 months) and 2.8 months for cohort 3 (95% CI, 2.7 to 3.0 months). In cohort 1, however, PFS was very comparable among TC3 or IC3 (5.6 months) and TC2/3 or IC2/3 groups (5.4 months). The updated median OS analysis was 23.5 months (26.9 months for TC3 or IC3 patients) for cohort 1; 15.5 months for cohort 2 and 13.3 months for cohort 3. The longest OS was 26.9 months (95% CI, 12.0 months to NE), which was observed in cohort 1 for the TC3 or IC3 group (32). These results seem to corroborate the preliminary results of another phase II study (FIR trial) which demonstrated a ORR of 29% in all PD-L1-selected treatment naïve, and in those with TC3 or IC3 tumors (33).
Durvalumab
Durvalumab, another PD-L1 inhibitor, is not FDA-approved for the treatment of metastatic NSCLC. Durvalumab has been evaluated in multiple settings in the treatment of NSCLC, including in the frontline. A phase 1/2 trial is ongoing and evaluates the safety and efficacy of single-agent durvalumab in patients with advanced NSCLC. In this study, durvalumab (10 mg/kg every 2 weeks) was administered to NSCLC treatment naïve patients. Initially, 15 patients were enrolled regardless of PD-L1 expression, and after a protocol amendment, enrollment was limited to PD-L1+ (≥25%). An updated preliminary analysis of safety and activity of 59 patients that received durvalumab was recently reported. All-grade treatment-related AEs were reported in 56%; and the most frequent were fatigue, diarrhea, and loss of appetite. Around 7% had a treatment-related AE leading to discontinuation of durvalumab, including diarrhea in 2 patients. Grade ≥3 treatment-related AEs was noticed in 10% and 1 patient died of drug-related pneumonia. For the PD-L1-high-expressors, ORR was 28.6% (95% CI, 16.6–43.3%); median PFS was 4.0 months (95% CI, 2.3–9.1); median OS was 21.0 months (95% CI, 14.5–NE); and 12-month OS rate was 72% (95% CI, 56–83%) (34,35). A phase 3 study is ongoing and compares durvalumab with platinum-based chemotherapy in treatment-naïve, PD-L1-high expressors (≥25%) with advanced NSCLC (NCT03003962). The MYSTIC trial is another phase 3 study that compares durvalumab with or without tremelimumab to platinum-doublet chemotherapy in the front-line setting for advanced NSCLC (NCT02453282). Preliminary data reported by the study sponsor failed to demonstrate a PFS benefit, which was the primary endpoint of the study.
Avelumab
Avelumab is a PD-L1 inhibitor that has been recently approved for the treatment of metastatic Merkel cell carcinoma (36). Avelumab is not currently indicated for the treatment of NSCLC. Results from a phase 1b trial evaluating the safety and clinical activity of avelumab as first-line treatment for advanced NSCLC were recently presented. A total of 145 patients with treatment naïve NSCLC, without driver mutations, and not preselected for PD-L1 expression, received avelumab 10 mg/kg IV every 2 weeks. Eighty two patients (56.6%) had a treatment-related AE; those occurring in ≥10% were infusion-related reaction (16.6%) and fatigue (14.5%).Thirteen patients (9.0%) had a grade ≥3 treatment-related AE. Four patients (2.8%) had a potential immune mediated treatment-related AE, all grade 1–2 (pneumonitis, 2.1%; hypothyroidism, 0.7%). No treatment-related deaths were observed in the trial. Unconfirmed ORR was 18.7% (95% CI, 10.6–29.3%) in 75 patients with ≥3 months’ of follow-up (37). A phase 3 trial (JAVELIN Lung 100), comparing avelumab to platinum-doublet in first-line NSCLC-PD-L1+ tumors is ongoing (NCT02576574).
Combination of checkpoint inhibitors with chemotherapy
Preclinical studies has shown that chemotherapy has the capability to (I) increase the expression of PD-L1 (antigenicity); (II) increase immunogenicity; and (III) enhance the capability of cancer cells to be recognized by the immune system (susceptibility) (38). This rational has led to multiple studies that are investigating the combination of chemotherapy and immunotherapy, including in the frontline setting for NSCLC.
In May of 2017, pembrolizumab received accelerated FDA approval for use in combination with carboplatin and pemetrexed for patients with treatment-naive metastatic NSCLC- adenocarcinoma, irrespective of PD-L1 expression. The approval was based on the results of a cohort of the phase 2 study (KEYNOTE-021), that randomized 123 patients to carboplatin/pemetrexed or to carboplatin/pemetrexed/pembrolizumab for 4 cycles followed by maintenance pembrolizumab/pemetrexed for a maximum of 24 months. The overall RR was 55% (95% CI, 42–68%) in the combination group versus 29% (95% CI, 18–41%) in the chemotherapy alone group. Interestingly, patients with PD-L1 expression (<1%) had a RR of 57%; those with expression ≥1% had a RR of 54%; and patients who had ≥50% had a RR of 80%. Median time to response was 1.5 months with pembrolizumab plus chemotherapy versus 2.7 with chemotherapy alone. Median PFS was 13.0 months (95% CI, 8.3 to not reached) for the combination and 8.9 (4.4–10.3) months for chemotherapy alone. The frequency of grade 3–4 treatment-related AEs was higher in the pembrolizumab arm (39% vs. 26%) (39). The results of the KEYNOTE-021 are promising; however, it needs to be validated in phase 3 setting (KEYNOTE-189 is ongoing—NCT02578680). The combination arm appears to be a good treatment approach for patients with bulky and or symptomatic disease that requires a rapid tumor response.
The phase I CheckMate 012 study assessed the effects of nivolumab monotherapy and nivolumab combined with platinum-based chemotherapy as first-line treatments in patients with advanced NSCLC. The nivolumab monotherapy cohort has been discussed previously in this review. In the combination cohort, a total of 56 patients with locally advanced or metastatic disease received nivolumab 5 or 10 mg/kg plus a platinum-based doublet every 3 weeks for 4 cycles followed by nivolumab maintenance until disease progression. The primary end point of the study was to evaluate safety and tolerability. Forty-five percent of patients experienced grade 3 or 4 treatment-related AEs; 7% of patients had pneumonitis; and 21% discontinued therapy due to treatment-related AEs. The ORRs ranged from 33% to 47% among the different chemotherapy regimens. ORRs were 48% and 43% for patients with ≥1% and <1% PD-L1 expression, respectively. The 24-week PFS rate ranged from 38% to 71% across the different chemotherapy arms, with a median PFS between 4.8 and 7.1 months. OS for the nivolumab 5 mg/kg plus carboplatin/paclitaxel arm was particularly noticeable; median OS was not reached (range, 8.8 to 30.1+ months), and 57% of patients were still alive after a median follow up time of more than 2 years. The 1-year OS rates ranged from 50% to 87% and were comparable to those seen in the nivolumab monotherapy cohort (73%) (40). Based on these results, the combination of nivolumab and platinum-based chemotherapy is tolerable and safe. Phase 3 studies are awaited in order to clarify whether the combination of nivolumab and chemotherapy improves OS compared to nivolumab alone. At this point, it is also unclear if PD-L1 expression has a role in predicting response to first-line treatment with nivolumab.
Atezolizumab has also been studied in combination with platinum-based chemotherapy in treatment naïve patients with NSCLC. A phase 1b enrolled a total of 37 patients to receive atezolizumab 15 mg/kg IV every 3 weeks combined with 4–6 cycles of carboplatin with either paclitaxel, pemetrexed, or weekly nab-paclitaxel. All chemotherapy regimens were followed by maintenance atezolizumab, if there was no evidence of disease progression. The primary end point was safety and efficacy of the combination. The most common treatment-related grade 3–4 AEs were neutropenia (36–42%), and anemia (16–31%). Three potentially related grade 5 AEs were seen (pneumonia; systemic candida; and autoimmune hepatitis). Confirmed RRs ranged between 36–64% across the three arms. Median PFS ranged from 5.7 to 8.4 months, and median OS from 12.9 to 19.3 months. Responses were seen in each arm independently of PD-L1 expression (41,42). With larger number of patients are needed to ratify the use of these combinations. The IMpower 132 trial is a large phase 3 trial, which is evaluating different chemotherapy regimens combined with atezolizumab in treatment-naïve patients with stage IV NSCLC (NCT02657434).
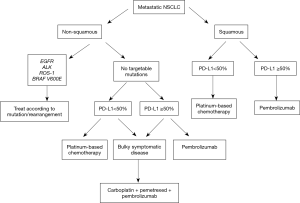
In summary, combination of immunotherapy plus chemotherapy has promising activity in the first-line setting of NSCLC. The combination appears particularly attractive to be used in NSCLC—adenocarcinoma patients with PD-L1 expression <50% and/or patients with bulky-symptomatic disease, where a rapid response is desired. For patients with PD-L1 expression ≥50%, single agent pembrolizumab is the new standard of care, at least until ongoing phase 3 combination studies are completed. Figure 1 summarizes a possible initial approach to patients with advanced NSCLC.
PD-1/PD-L1 plus CTL-4 inhibitors
The combination of nivolumab and ipilimumab led to impressive results in patients with metastatic melanoma. The 2-year OS with this combination in previously untreated patients with melanoma was 64% (43). Based on these impressive results, PD-1/PD-L1 plus CTLA-4 inhibitors are being tested in NSCLC. One cohort of the CheckMate-012 study discussed above, evaluated the combination of nivolumab and ipilimumab in the first-line setting for patients with advanced NSCLC. This cohort of the phase 1 assigned 78 patients to receive nivolumab 1 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks, nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 12 weeks, or nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks. The primary end point was safety and tolerability. The first cohort (nivolumab 1 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks) was not considered suitable for further clinical development. Treatment-related AEs of any grade were reported in 76% and 82% with ipilimumab every 12 weeks and ipilimumab every 6 weeks, respectively. Grade 3 and 4 AEs were 37% and 33%, respectively. The most commonly reported grade 3 or 4 treatment-related AEs were increased lipase, pneumonitis, adrenal insufficiency, and colitis. Treatment-related serious AEs were reported in 32% patients in the ipilimumab every-12-week cohort and 28% patients in the every-6-week cohort. Treatment-related AEs (any grade) prompted treatment discontinuation in 11% of the patients in the every-12-week cohort and 13% of the patients in the every-6-week cohort. There were no treatment-related deaths. RR was 47% with nivolumab plus ipilimumab every 12 weeks vs. 38% with nivolumab plus ipilimumab every 6 week. In patients with PD-L1 of ≥1%, RR was 57% in both cohorts, ipilimumab every-12-week and every-6-week. In patients with PD-L1 expression ≥1%, the RR was 57%; and in patients with ≥50%, the RR was 92%. OS at 1 year was 69% (95% CI, 52−81%) in the every-6-week cohort; however, it was not reported in the ipilimumab every-12-week cohort yet (44). CheckMate-227 is an ongoing phase 3 trial that randomizes patients to nivolumab, or nivolumab plus ipilimumab, or nivolumab plus chemotherapy (NCT02477826).
The other PD-1/PD-L1 plus CTLA-4 combination being tested upfront is durvalumab and tremelimumab. A phase 1b assessing the safety and activity of this regimen enrolled 102 patients in the dose-escalation phase. Durvalumab was administered at doses of 3, 10, 15, or 20 mg/kg every 4 weeks, or 10 mg/kg every 2 weeks, and tremelimumab at doses of 1, 3, or 10 mg/kg every 4 weeks for 6 doses then every 12 weeks for three doses. The maximum tolerated dose (MTD) was exceeded in the cohort receiving durvalumab 20 mg/kg every 4 weeks plus tremelimumab 3 mg/kg, with 30% of the patients having a dose-limiting toxicity (DLT) (one grade 3 increased aspartate aminotransferase and alanine aminotransferase and one grade 4 increased lipases). The most frequent treatment-related grade 3 and 4 AEs were diarrhea (11%), colitis (9%), and increased lipase (8%). Treatment-related AEs leading to treatment discontinuation occurred in 28%, and treatment-related serious AEs occurred in 36% patients. Three deaths thought to be related to treatment included complications arising from myasthenia gravis, pericardial effusion and neuromuscular disorder. RR was 23% in the combined tremelimumab 1 mg/kg cohort, and 20% in the combined tremelimumab 3 mg/kg cohort. Responses were noted in both, PD-L1-positive and PD-L1-negative tumors. No responses were reported in the durvalumab 15 mg/kg every 4 weeks plus tremelimumab10 mg/kg cohort (45). The NEPTUNE trial is an ongoing phase 3 study, comparing first-line durvalumab plus tremelimumab to platinum-doublet chemotherapy (NCT02542293). POSEIDON is a phase 3 trial, which compares durvalumab plus tremelimumab plus chemotherapy or durvalumab plus chemotherapy to chemotherapy alone as first line treatment for metastatic NSCLC (NCT03164616).
Other immunotherapy combinations
Epacadostat is a highly potent and selective inhibitor of indoleamine-2-3-dioxygenase-1 (IDOI). IDOI is an important enzyme that catalyzes the degradation of the amino acid tryptophan to N-formyl-kynurenine, inhibiting antitumor immune response (46). A phase 1/2 dose escalation study (ECHO-202/KEYNOTE-037) evaluated epacadostat plus pembrolizumab after platinum-based chemotherapy in patients with advanced NSCLC. Treatment was well tolerated with promising activity, with a RR of 35% in the second-line setting (47). ECHO-207 is an ongoing phase 1/2 multicohort dose-escalation trial assessing the safety and tolerability of epacadostat given in combination with a PD-1 inhibitor (nivolumab or pembrolizumab) and platinum-based chemotherapy in the first-line setting (NCT03085914).
Bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor is approved for upfront use with platinum-based chemotherapy in patients with metastatic adenocarcinoma. IM power150 is a phase 3 trial comparing atezolizumab in combination with carboplatin/paclitaxel with or without bevacizumab to carboplatin/paclitaxel plus bevacizumab in patients with stage IV non-squamous cell carcinoma (NCT02366143).
The PARP inhibitor veliparib is also being tested upfront in combination with nivolumab and platinum-doublet chemotherapy in a phase 1 dose-escalation and phase 2 randomized trial (NCT02944396).
Unresolved issues
As discussed above, the checkpoint inhibitors have revolutionized the treatment of NSCLC. However, a number of questions remain. The optimal patient selection strategies for these agents are unclear. While it is clear that high PD-L1 expression is associated with a higher RR, even patients with low or absent PD-L1 expression have demonstrated responses as well (22). Tumor mutational load has also been proposed as a potential predictive marker for response to these agents, but it has not yet been tested prospectively (28,29).
Another question that arises is that of the reproducibility of the PD-L1 testing. Currently there are four different assays for testing PD-L1 expression: PD-L1 IHC 22C3 for pembrolizumab, PD-L1 IHC 28-8 for nivolumab (both on the Dako platform), SP142 assay for atezolizumab and SP263 assay for durvalumab (both on the Ventana platform). In the IASLC Blueprint PD-L1 IHC Assay Comparison Project, 36.9% of the cases showed discrepancies in PD-L1 expression between the assays, leading to a misclassification of PD-L1 status (48).
Conclusions
There has been an enormous amount of research investigating the different combinations of PD-1/PD-L1, CTLA-4 and chemotherapy in the front-line setting. Pembrolizumab is the current standard of care for patients with metastatic NSCLC, whose tumors have PD-L1 expression ≥50%, and no targetable drivers. For those patients with PD-L1 expression <50%, platinum-based chemotherapy is still the standard of care. However, the combination of carboplatin, pemetrexed and pembrolizumab is a reasonable alternative for non-squamous cell histology with bulky and symptomatic disease that needs a fast treatment response, independently of PD-L1 expression. Interestingly, however, nivolumab monotherapy and nivolumab in combination with chemotherapy (based on a phase I study) failed to demonstrate a PFS and survival benefit in comparison to platinum-based chemotherapy in the first-line setting, irrespective of PD-L1 expression. It remains unclear the reason of this discrepancy. The combination of PD-1/PD-L1 plus a CTLA-4 inhibitor has also revealed impressive responses regardless of PD-L1 expression; nevertheless, at the expense of more toxicity. Perhaps, this could be another attractive first-line regimen for patients with good performance status and low PD-L1 expression.
How best to combine new immunotherapies is a challenging question. At this point, the data is still premature making it difficult to make further conclusions. It is possible that PD-1/PD-L1 inhibition alone is adequate for high-PD-L1 expressors and that a combination regimens (PD-1/PD-L1 plus chemotherapy, or PD-1/PD-L1 plus CTLA-4) may be required for unselected patients, and those with PD-L1 <50%. Hopefully, the result of phase 3 trials will help elucidate these questions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Azzoli CG, Baker S Jr, Temin S, et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 2009;27:6251-66. [Crossref] [PubMed]
- Azzoli CG, Temin S, Aliff T, et al. 2011 Focused Update of 2009 American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small-Cell Lung Cancer. J Clin Oncol 2011;29:3825-31. [Crossref] [PubMed]
- NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol 2008;26:4617-25. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [Crossref] [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012;30:2055-62. [Crossref] [PubMed]
- Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004;22:2184-91. [Crossref] [PubMed]
- Paz-Ares L, Mezger J, Ciuleanu TE, et al. Necitumumab plus pemetrexed and cisplatin as first-line therapy in patients with stage IV non-squamous non-small-cell lung cancer (INSPIRE): an open-label, randomised, controlled phase 3 study. Lancet Oncol 2015;16:328-37. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Brahmer JR, Pardoll DM. Immune checkpoint inhibitors: making immunotherapy a reality for the treatment of lung cancer. Cancer Immunol Res 2013;1:85-91. [Crossref] [PubMed]
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. [Crossref] [PubMed]
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793-800. [Crossref] [PubMed]
- Disis ML. Mechanism of action of immunotherapy. Semin Oncol 2014;41 Suppl 5:S3-13. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2980-7. [Crossref] [PubMed]
- Socinski M, Creelan B, Horn L, et al. CheckMate 026: A phase 3 trial of nivolumab vs investigator’s choice (IC) of platinum-based doublet chemotherapy (PT-DC) as first-line therapy for Stage IV/recurrent programmed death ligand 1 (PD-L1) − positive NSCLC. Ann Oncol 2016.27.
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Peters S, Gettinger S, Johnson ML, et al. Phase II Trial of Atezolizumab As First-Line or Subsequent Therapy for Patients With Programmed Death-Ligand 1-Selected Advanced Non-Small-Cell Lung Cancer (BIRCH). J Clin Oncol 2017;35:2781-9. [Crossref] [PubMed]
- Spigel DR, Chaft JE, Gettinger SN, et al. Clinical activity and safety from a phase II study (FIR) of MPDL3280A (anti-PDL1) in PD-L1–selected patients with non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33:8028.
- Antonia SJ, Kim SW, Spira AI, et al. Safety and clinical activity of durvalumab (MEDI4736), an anti-PD-L1 antibody, in treatment-naïve patients with advanced non-small-cell lung cancer. J Clin Oncol 2016;34:9029.
- Antonia SJ, Brahmer JR, Balmanoukian AS, et al. Safety and clinical activity of first-line durvalumab in advanced NSCLC: Updated results from a phase 1/2 study. J Clin Oncol 2017;35:e20504.
- Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol 2016;17:1374-85. [Crossref] [PubMed]
- Jerusalem G, Chen F, Spigel D, et al. JAVELIN solid tumor: Safety and clinical activity of avelumab (anti-PD-L1) as first-line treatment in patients with advanced NSCLC. J Thorac Oncol 2017;12:S252. [Crossref]
- Galluzzi L, Buqué A, Kepp O, et al. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015;28:690-714. [Crossref] [PubMed]
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2969-79. [Crossref] [PubMed]
- Liu SV, Powderly JD, Camidge DR, et al. Safety and efficacy of MPDL3280A (anti-PDL1) in combination with platinum-based doublet chemotherapy in patients with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33:8030.
- Liu SV, Camidge DR, Gettinger SN, et al. Atezolizumab (atezo) plus platinum-based chemotherapy (chemo) in non-small cell lung cancer (NSCLC): Update from a phase ib study. J Clin Oncol 2017;35:9092.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Overall survival results from a phase III trial of nivolumab combined with ipilimumab in treatment-naïve patients with advanced melanoma (CheckMate-067). Cancer Res 2017;77:abstr CT075.
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31-41. [Crossref] [PubMed]
- Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 2016;17:299-308. [Crossref] [PubMed]
- Beatty GL, O’Dwyer PJ, Clark J, et al. First-in-Human Phase I Study of the Oral Inhibitor of Indoleamine 2,3-Dioxygenase-1 Epacadostat (INCB024360) in Patients with Advanced Solid Malignancies. Clin Cancer Res 2017;23:3269-76. [Crossref] [PubMed]
- Gangadhar TC, Schneider BJ, Bauer TM, et al. Efficacy and safety of epacadostat plus pembrolizumab treatment of NSCLC: Preliminary phase I/II results of ECHO-202/KEYNOTE-037. J Clin Oncol 2017;35:abstr 9014.
- Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol 2017;12:208-22. [Crossref] [PubMed]