Hong Kong Med J 2019 Dec;25(6):460–7 | Epub 4 Dec 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Associations of clinical and dosimetric parameters with
late rectal toxicities after radical intensity-modulated radiation therapy
for prostate cancer: a single-centre retrospective study
Brian YH Ng, MB, ChB, FRCR1; Ellen LM
Yu, BSc, MSc2; Tracy TS Lau, MB, BS, FHKCR1; KS Law,
MB, BS, FHKCR1; Ashley CK Cheng, MB, BS, FHKCR1
1 Department of Oncology, Princess
Margaret Hospital, Laichikok, Hong Kong
2 Clinical Research Centre, Princess
Margaret Hospital, Laichikok, Hong Kong
Corresponding author: Dr Brian YH Ng (bryan.yh.ng@gmail.com)
Abstract
Introduction: This study
assessed the incidence of late rectal toxicities and evaluated potential
predictive factors for late proctitis in patients treated with
prostate-specific intensity-modulated radiotherapy in Hong Kong.
Methods: This retrospective
longitudinal observational study included patients with localised
prostate cancer who were treated with intensity-modulated radiation
therapy in an oncology unit in Hong Kong between January 2007 and
December 2011, and who had >1 year of follow-up. Clinical,
pharmacological, and radiation parameters were recorded. Toxicities were
measured by Common Terminology Criteria for Adverse Events version 4.
Results: In total, 232 patients
were included in this analysis. The mean follow-up time was 7.3 ± 2.1
years and 46.5% of the patients had late rectal toxicities. Late
proctitis occurred in 30.5% of patients; 25% of the patients with late
proctitis exhibited grade ≥2 toxicity. Median onset times for late
proctitis and rectal bleeding were 15 and 18.4 months, respectively.
Multivariable regression showed increased odds for the occurrence of
late proctitis in patients with older age (odds ratio [OR]=1.11, 95%
confidence interval [CI]=1.04-1.19, P=0.003), higher V70 (OR=1.08, 95%
CI=1.01-1.15, P=0.027), and presence of acute rectal toxicities
(OR=4.47, 95% CI=2.37-8.43, P<0.001). Antiplatelet use was not
significantly associated with the occurrence of late proctitis (OR=1.98,
95% CI=0.95-4.14, P=0.07).
Conclusions: The incidence of
late rectal toxicities was considerable among patients in this study.
Clinicians should consider the possibility of late proctitis for
patients with older age, acute rectal toxicities, and higher V70. High
doses to rectal volumes should be limited because of the significant
association with V70.
New knowledge added by this study
- Age, V70, and the presence of acute rectal toxicities were identified as potential predictive factors for the occurrence of late proctitis in prostate cancer patients who undergo treatment with intensity-modulated radiotherapy.
- This is the first study in Hong Kong to describe the incidence of late rectal toxicities over time and to identify associations between pharmacological factors and the occurrence of late proctitis in patients with prostate cancer who undergo treatment with intensity-modulated radiotherapy with radical intent.
- Clinicians should closely monitor patients for the development of late rectal toxicities, including proctitis, following intensity-modulated radiotherapy for prostate cancer.
- Clinicians should promptly investigate any rectal symptoms that develop after radiotherapy in patients who exhibit factors predictive of high risk, including older age, the presence of acute rectal toxicities, and higher V70.
- During radiotherapy planning for patients with prostate cancer, clinicians should attempt to limit the applications of high doses to rectal volumes.
Introduction
Radical radiotherapy is a standard treatment option
for patients with early-stage and locally advanced non-metastatic prostate
cancer. Advances in radiotherapy in the past 20 years include the use of
androgen deprivation therapy for patients with this type of cancer, as
well as the application of more precise radiotherapy techniques.1 2
Intensity-modulated radiation therapy (IMRT) has emerged as the standard
radiotherapy technique.3 Its
benefits have been explored in terms of the effects of dose escalation or
hypofractionation on survival outcomes.4
5 For patients undergoing this type
of treatment, toxicities are the primary concern. Long-term side-effects
(ie, complications occurring ≥3 months after radiotherapy) have a major
impact on the quality of life for affected patients; this is particularly
important for patients with genitourinary or rectal toxicities. Late
rectal toxicities, including per-rectal bleeding, faecal incontinence, and
proctitis, have been reported to occur at rates of 5% to 21%.1 34 5 6 7 8 9
Associations have been reported between late rectal
toxicities and various clinical and dosimetric parameters; however, most
data were collected using the conventional three-dimensional conformal
technique.8 1011 12 In addition, there have been limited reports of such
associations among patients in Hong Kong. In particular, Poon et al8 reported that 8% of patients exhibited grade ≥2 late
rectal toxicities following IMRT in a retrospective cohort study. Although
several clinical parameters were assessed, most failed to show
statistically significant associations, with the exception of the presence
of acute rectal toxicities.8 To the
best of our knowledge, pharmacological parameters following IMRT for
prostate cancer have not yet been studied in local populations. Some
previous reports showed a significant association between anticoagulant
use and late rectal toxicities, whereas an association between
antiplatelet use and androgen deprivation was inconsistent among studies.12 13
14
Multiple strategies have been used for the
treatment of late rectal toxicities. The use of hyperbaric oxygen has
shown promising results in some retrospective studies, but it has not been
available in Hong Kong until recently.12
15 16
Treatments with sucralfate, prednisolone enaema, short-chain fatty acids,
and antifibrinolytics have been evaluated in small trials.17 18 19 Thus far, no standard approach has been established,
and there are no published data regarding local management practices.
Late rectal toxicities may represent clinically
significant complications because of their non-negligible incidences.
Insights regarding any factors predictive of their occurrence could aid in
improved treatment planning and early identification of toxicity. This
study was performed to assess the incidence of late rectal toxicities and
to identify factors predictive for late proctitis in patients treated with
prostate-specific IMRT in Hong Kong.
Methods
Study design and patients
This retrospective longitudinal observational study
included patients with prostate cancer who received IMRT with radical
intent in a tertiary referral institution in Hong Kong from January 2007
to December 2011. Patients were excluded if they were followed up for
fewer than 12 months from the start of radiotherapy, if they did not
complete the course of radiotherapy, if they were not at risk of proctitis
(eg, those with post-abdominoperineal resection), or if they did not have
a retrievable radiotherapy plan due to technical difficulties. The cut-off
date for data collection was 31 December 2018.
Patients underwent treatment with a comfortably
full bladder and an empty rectum, with laxatives administered 1 day prior
to simulation computed tomography. Patients were asked to empty the
bladder prior to attending the radiotherapy suite, and then drink a
comfortable volume of water. A pelvic thermoplastic mould was used for
immobilisation. Intravenous contrast was administered prior to computed
tomography. Re-simulation was performed automatically if bladder volume
was below 150 cc, if prominent rectal gas was present, or upon request by
the attending oncologist. Contouring was performed by designated
oncologists with confirmation by at least one specialist. Tumour and whole
prostate were contoured as a single volume; the clinical target volume
(CTV) was the volume of the tumour, whole prostate, and base of the
seminal vesicle (defined as 1 cm of the central seminal vesicle proximal
to the base of the prostate). Whole seminal vesicle was included in the
CTV if seminal vesicle involvement was observed. Planning target volume
(PTV) was determined by expanding the CTV by a radial margin of 1.5 cm,
except posteriorly where a smaller margin was used (0.7 cm). Pelvic lymph
node irradiation was not performed. Patients received 70 Gy in 35 daily
fractions over 7 weeks at 100% of the isodose level. Rectal volume was
contoured in accordance with the Radiation Therapy Oncology Group
Consensus Contouring Guidelines for normal male pelvic tissue. Dose
constraints for organs at risk followed our departmental protocol: for the
rectum, we classified the plan as fulfilling the first, second, or third
criteria. First criteria were satisfied if V40 (% of organ volume
receiving 40 Gy) <35% or V65 (% of organ volume receiving 65 Gy)
<17%; second criteria were satisfied if V53 (% of organ volume
receiving 53 Gy) <45% or V68 (% of organ volume receiving 68 Gy)
<20%; and third criteria were satisfied if V60 (% of organ volume
receiving 60 Gy) <50%, V65 (% of organ volume receiving 65 Gy) <35%,
or V70 (% of organ volume receiving 70 Gy) <25%. Hormonal treatment was
administered based on the risk stratification used in the United Kingdom
National Institute for Health and Care Excellence guidelines. Patients
were followed up at 3–6-month intervals until the patient died or
defaulted, and data were censored at the last recorded follow-up. Dose
distributions, doses administered to organs at risk, and dose volume
histograms were evaluated by the Eclipse and Planning System (Varian
Medical Systems; Palo Alto [CA], United States).
Data collection
For each patient, basic demographic data were
documented, including age; Eastern Cooperative Oncology Group performance
score; smoking habit; pretreatment albumin level; co-morbidities such as
hypertension, diabetes, lipid disorder, history of cerebrovascular
disease, ischaemic heart disease, and/or chronic renal impairment; medical
history of abdominal surgery; drug history including antihypertensives,
oral glycaemic agents, antiplatelets, anticoagulants, lipid-lowering
agents, and antipurine agents; androgen deprivation therapies, including
medical or surgical castration; and use of immunosuppressants. Tumour
characteristics were also recorded, including pretreatment
prostate-specific antigen level, clinical T-staging determined by clinical
and radiological findings (based on AJCC 7th edition20), and Gleason score.
Acute and late rectal toxicities, including
proctitis, incontinence, and per-rectal bleeding, were recorded and
classified in accordance with Common Terminology Criteria for Adverse
Events version 4.21 Late rectal
toxicities were defined as those that occurred at least 3 months after the
completion of radiotherapy. Late proctitis was defined as either the
presence of rectal symptoms listed in Common Terminology Criteria for
Adverse Events version 4, or colonoscopy findings of proctitis (eg,
telangiectasia, ulcers, or inflammation). If a patient presented with
per-rectal bleeding, colonoscopy findings were referenced whenever present
to differentiate proctitis or other causes of bleeding, such as
diverticulosis or haemorrhoids. Per-rectal bleeding only was recorded if
no endoscopic proctitis features were present; otherwise, both per-rectal
bleeding and proctitis were recorded. Additional parameters recorded
included time of onset of late rectal toxicities, as well as treatment
modalities used.
Dosimetric parameters (eg, V40, V50, V60, V70, Dmax
[maximum dose], mean dose to rectum, and contoured rectal volume) were
evaluated with the radiotherapy planning system. The use of static beam or
volumetric arc technique was recorded, as was the compliance with rectal
dose constraints.
Statistical analysis and research ethics
Incidences of grade ≥1 late rectal toxicities with
95% confidence interval (CI) were calculated at 1, 2, and 5 years after
treatment. The Kaplan-Meier curve method was used to illustrate the time
to onset of late rectal toxicities. The Chi squared test, Fisher’s exact
test, independent t test, or Mann-Whitney U test were used
to compare baseline patient characteristics, pharmacological and
dosimetric parameters between patients in grades 0 and ≥1 late toxicities,
as well as in patients with late proctitis. The association of each
parameter with late proctitis was examined using a multivariable binary
logistic regression model with a backward stepwise selection method,
including variables with P<0.1 in univariable regression analyses. The
presence of multicollinearity was determined by using variance inflation
factors. Statistical analyses were performed using SPSS (Windows version
22.0; IBM Corp, Armonk [NY], United States). The threshold of statistical
significance was set at P<0.05. The STROBE checklist was followed to
ensure standardised reporting.
Results
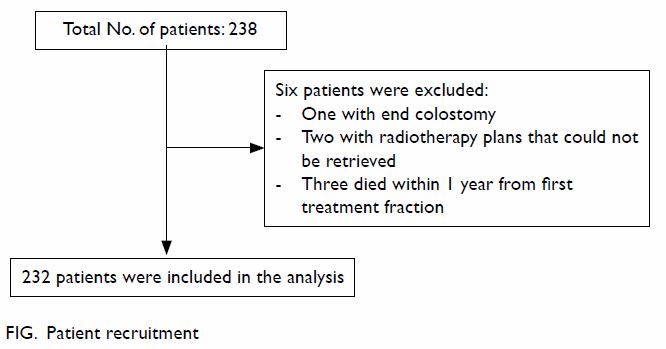
From January 2007 to December 2011, a total of 238
patients with prostatic cancer received radical radiotherapy in our
institution. As shown in the Figure, 232 patients were included in the analysis.
The mean age of patients was 72.3 ± 4.8 years at time of radiotherapy (Table 1). The mean follow-up period was 7.3 ± 2.1
years, and there were 157 (67.7%) surviving patients at the cut-off date
for data collection. Forty-two (18.1%) patients had been diagnosed with
biochemical recurrence during the study period, based on the Phoenix
definition.22 In total, 229
patients received a PTV dose of ≤70 Gy. Owing to genuine bowel invasion,
or as a component of individualised dose escalation, four patients
received a PTV dose of 66 to 76 Gy, of which three were >70 Gy.
Colonoscopy was performed in 103 (44.4%) patients during follow-up. Among
patients with per-rectal bleeding, 93 (88.6%) had undergone colonoscopy.
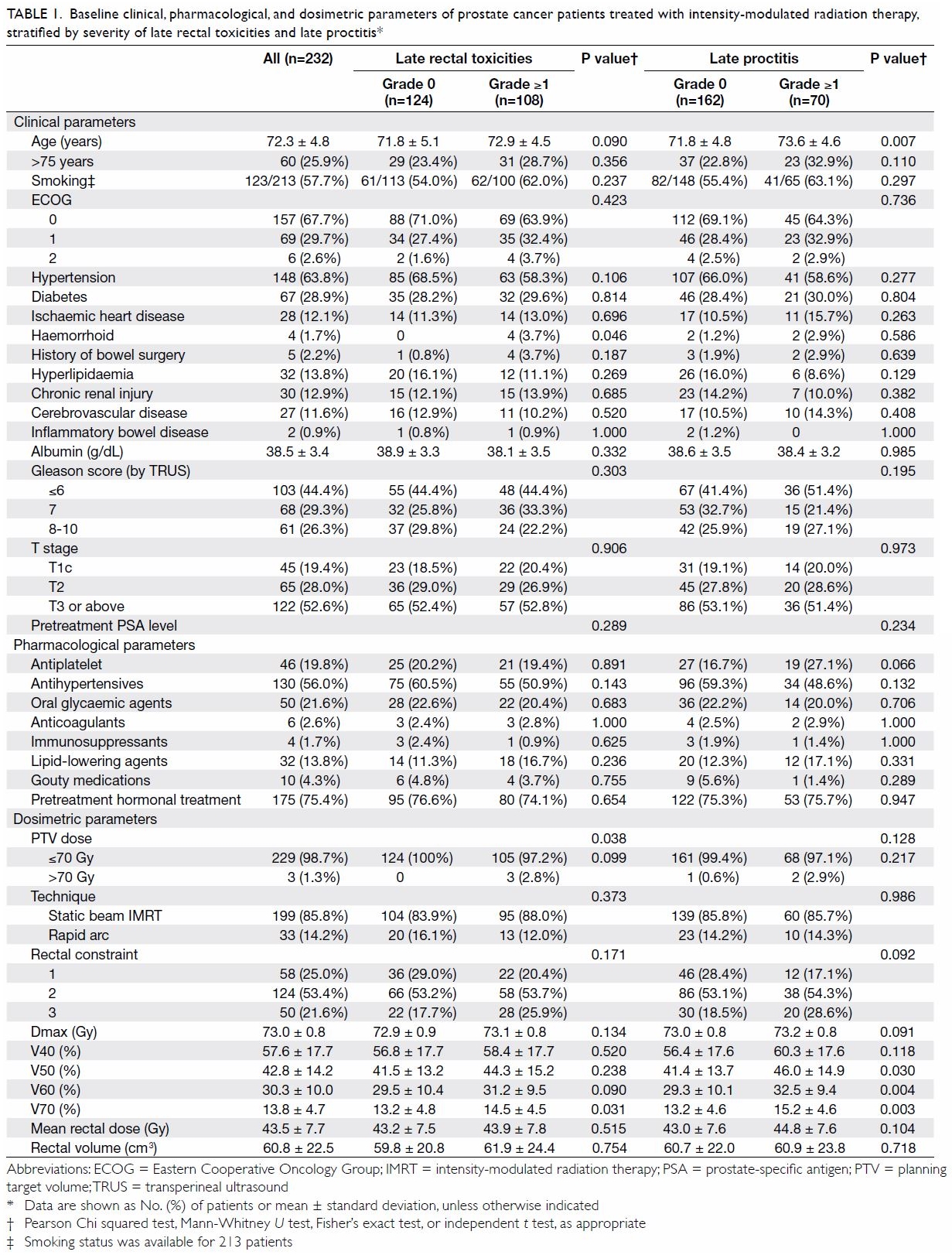
Table 1. Baseline clinical, pharmacological, and dosimetric parameters of prostate cancer patients treated with intensity-modulated radiation therapy, stratified by severity of late rectal toxicities and late proctitis
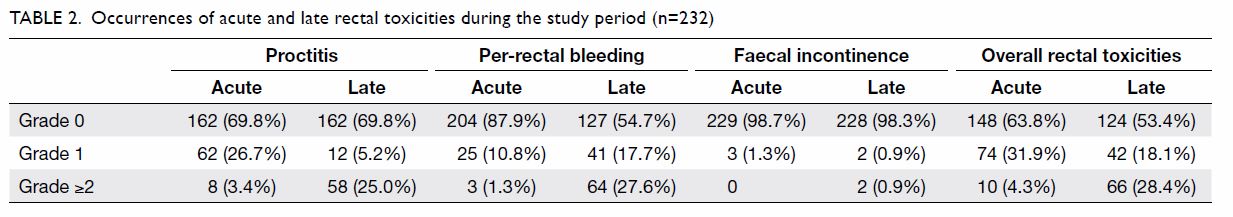
Occurrences of acute and late rectal toxicities
throughout the study period are shown in Table 2. The rates of all-grade acute and late
rectal toxicities were 36.2% and 46.5%, respectively; the rates of grade
≥2 late rectal toxicities and proctitis were 28.4% and 25.0%,
respectively. Nineteen (8.2%) patients had grade 3 per-rectal bleeding,
with 15 (78.9%) requiring blood transfusion and eight (42.1%) requiring
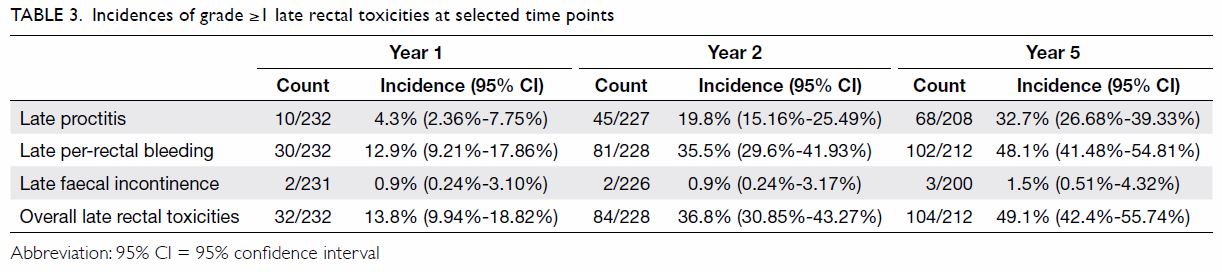
endoscopic coagulation. The cumulative incidences of rectal toxicities at
1, 2, and 5 years after treatment are shown in Table 3. The median times of onset of late
proctitis, late faecal incontinence, and late per-rectal bleeding were 15,
21.8, and 18.4 months, respectively.
Patients’ detailed demographic, pharmacological,
and dosimetric parameters are listed in Table 1. Factors including history of haemorrhoid,
PTV dose, and V70 were significantly different between patients with and
without late rectal toxicities. In addition, age was the sole demographic
factor significantly associated with late proctitis. There was no
significant association between antiplatelet use and late rectal
toxicities (P=0.066). No associations were found between late proctitis
and other demographic or pharmacological characteristics (eg, PTV dose and
history of haemorrhoid) in this study.
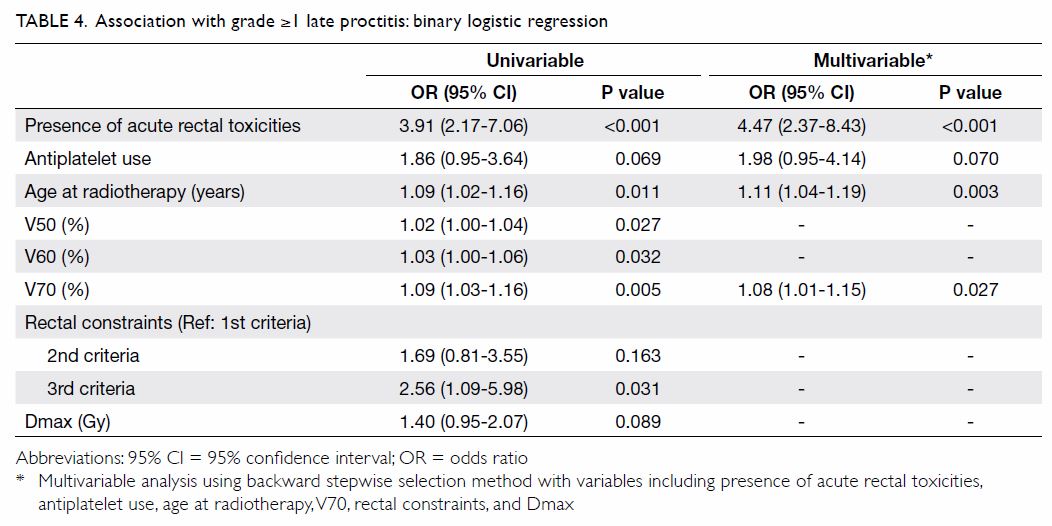
Univariable and stepwise multivariable analyses
were performed to identify factors predictive of
late proctitis (Table 4). In univariable analysis, the presence of
acute rectal toxicities, antiplatelet use, age at radiotherapy, Dmax, and
dose/volume histogram parameters (ie V50, V60, V70, and rectal
constraints) were identified as potential risk factors. In the regression
model with all potential risk factors included, multicollinearity was
detected among the dose/volume histogram parameters (variance inflation
factors of 7.21, 8.69, 3.05, and 4.97 for V50, V60, V70, and rectal
constraints, respectively). Compared to V50 and V60, V70 (ie, the
high-dose region) showed a stronger association with late proctitis in
univariable analysis. Multicollinearity was resolved by exclusion of V50
and V60 from the multivariable regression model. The final multivariable
regression model revealed increased odds of late proctitis in patients
with older age, higher V70, and the presence of acute rectal toxicities.
Antiplatelet use tended to show higher odds, but this finding was not
statistically significant (odds ratio=1.98, 95% CI=0.95-4.14). Dmax and
satisfaction of the 3rd criteria alone were associated with late proctitis
in univariable analysis, but the associations were not significant in
multivariable analysis.
Common treatment modalities among patients with
grade ≥2 late proctitis were also recorded. Topical agents such as
Ultraproct® (commercial preparation of fluocortolone pivalate,
fluocortolone hexanoate, and cinchocaine hydrochloride), bismuth ointment,
or an antifibrinolytic agent (eg, tranexamic acid) are commonly used as
first-line treatment.23 More than
half (53.4%) of the patients had been administered an antifibrinolytic
agent, while 77.6% and 19% of the patients were prescribed Ultraproct® and
bismuth, respectively. Prednisolone enaema was also administered in 22
(37.9%) patients; the median duration of enaema use was 3.5 months
(interquartile range, 1-7.25 months). Subjective improvement was reported
by eight (36.4%) patients who received enaema treatment.
Discussion
Radiation proctitis and other long-term rectal
toxicities are clinically significant complications of radiotherapy to the
prostate, due to their detrimental effects on patients’ quality of life,
as well as the expected long duration of post-treatment survival. In our
cohort, the incidences of late proctitis (30.2%) and overall rectal
toxicities (46.5%) were slightly higher than those in previous reports
(5%-21%).1 3 4 5 6 7 8 9 Comparison of baseline characteristics showed that more
patients had ≥T3 disease in our cohort, although we found no statistically
significant association between T-staging and a higher incidence of
proctitis; similarly, no association between these parameters were
reported in other studies.8 12 Other variables with possible interactions were
similar between our study and prior studies; these included age,
dosimetric parameters (eg, V70, which was 14% in our study and 10% to 23%
in previous studies), and the use of antiplatelets.8 11 12
There are two possible explanations for the higher
incidences of late proctitis and overall rectal toxicities. First, our
study involved frequent utilisation of colonoscopy for any rectal
symptoms, which may lead to a higher rate of recognition; notably, the
rate of utilisation was not reported in previous studies. Second, our
study had a relatively long follow-up period. Previous studies described
the incidence of toxicity throughout the study period. The mean follow-up
period in our study was 7.3 years, whereas that of most previous studies
was 38.9 to 66 months; in one notable exception, the follow-up period was
8.4 years (the incidence was 21% in that study).3
The longer study period may also have contributed to a higher number of
late rectal toxicities.
Previous reports suggested that a variety of
parameters are associated with late proctitis; knowledge of these
parameters could help clinicians to predict the risk of proctitis in each
patient. In our study, age, and dosimetric parameters including V50, V60,
and V70 were associated with late proctitis; history of haemorrhoid and
V70 were associated with overall late rectal toxicities. These findings
are consistent with the results of previous studies.10 11 12 13 14 24 Some
factors identified in prior studies, including diabetes, previous
abdominal surgery, and the use of antiandrogen or anticoagulant
medication,11 13 25 failed to
demonstrate any associations in the present study. Of note, <10% of the
patients in our study had a history of abdominal surgery or inflammatory
bowel disease; this could have influenced our ability to identify a
statistically significant association. Recall bias, incomplete
documentation of coexisting medical conditions and pharmacological
histories, and the relatively small sample size in our cohort may have
influenced our conclusions regarding factors associated with overall late
rectal toxicities and/or late proctitis.
Several dosimetric parameters and dose/volume
histogram data (including V50, V60, and V70) were also associated with
late proctitis, as in previous studies.8
Our in-house rectal constraints did not demonstrate significant
associations with the occurrence of proctitis (P=0.092). Notably, in the
present study, the PTV dose was associated with overall late toxicities,
but not with late proctitis specifically. Most patients received 70 Gy in
this study; therefore, the effects of PTV dose on complications were
difficult to establish.
Regression analysis was used to predict the odds of
late proctitis among patients in our study. As shown in Table
4, higher V70, older age, and the presence of acute rectal
toxicities were found to increase the odds of late proctitis. Poon et al8 also reported similar findings
concerning acute rectal toxicities; however, they did not find
associations with V70 or age. The increased incidence of late proctitis in
our study may have enhanced our ability to identify significantly
associated factors. Nevertheless, both our present study and the study of
Poon et al8 demonstrated that
patients with acute rectal toxicities during radiotherapy had higher
incidences of late proctitis than patients without acute rectal
toxicities. Similar results were reported by Fellin et al.11 Taken together, the present and prior results
indicate that the presence of acute toxicities is predictive for late
proctitis. Clinicians should be vigilant and perform prompt investigations
when patients with acute toxicities report any rectal symptoms during
subsequent follow-up.
Theoretically, dosimetric parameters are expected
to be associated with late proctitis. In our study, the dosimetric
parameters exhibited modest associations with late proctitis. Notably, we
did not find a significant association between our in-house rectal
constraints and the occurrence of late proctitis. Fellin et al11 demonstrated similar associations between late
proctitis and V70, as well as other dosimetric parameters, in their
cohort. This suggests that the presence of confounding factors may reduce
the strength of associations with late proctitis. A notable factor is the
inter-fractional variation of rectal and bladder filling; specifically,
Miralbell et al26 found that
rectal filling was significantly associated with late rectal toxicities.
Imaging-guided radiotherapy with inter-fractional bowel and bladder
control has been suggested in accordance with the nomogram designed by
Delobel et al9; this type of
therapy could reduce the risks of acute and late rectal toxicities. In our
study, there was no strict inter-fractional bowel or imaging control for
bladder and rectal volumes during the course of IMRT. Although we found no
statistically significant difference in the mean rectal volume during
simulation computed tomography between patients with and without late
proctitis, we could not retrieve the inter-fractional variation in rectal
volumes for analysis in this study; this factor was also excluded from
analysis in the study by Fellin et al.11
Although identical instructions were provided to patients during
simulation and treatment, inter-fractional variations may have been
statistically significant. To further confirm whether dosimetric
parameters are predictive of late proctitis, a prospective study is needed
in which strict interfractional rectal and bladder control are performed,
in combination with improved treatment verification strategies (eg, the
use of cone beam computed tomography).
There were a few weaknesses in this study. First,
this was a retrospective study in which incomplete reporting may have
occurred and data might have been missing. Second, the small sample size
and the low prevalences of some clinical factors and events may have
affected the statistical power to determine associations between rates of
complications and potential predictive factors (eg, use of anticoagulants
and presence of inflammatory bowel disease). Third, confounding factors
might have been present as mentioned earlier in the Discussion, and could
not be controlled because of the retrospective nature of this study.
However, this study did identify factors that clinicians could use to
predict the occurrence of late proctitis. The significant association of
V70 with late proctitis should be applied to radiotherapy planning, in
that high doses to the rectal volume should be limited where possible.
In summary, late rectal toxicities were frequent
among patients in this study in Hong Kong. The occurrence of late
proctitis was associated with age, V50, V60, and V70; the occurrence
overall late rectal toxicities was associated with a history of
haemorrhoid, PTV dose, and V70. Multivariable regression analysis
suggested that age, V70, and the presence of acute rectal toxicities could
predict the occurrence of late proctitis. Clinicians should closely
monitor patients for the occurrence of late proctitis if they exhibit
these high-risk factors.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept or design: BYH Ng, ACK Cheng.
Acquisition of data: BYH Ng.
Analysis or interpretation of data: BYH Ng, ELM Yu, TTS Lau.
Drafting of the article: BYH Ng, ELM Yu, TTS Lau, KS Law.
Critical revision for important intellectual content: BYH Ng, ELM Yu, KS Law, ACK Cheng.
Acquisition of data: BYH Ng.
Analysis or interpretation of data: BYH Ng, ELM Yu, TTS Lau.
Drafting of the article: BYH Ng, ELM Yu, TTS Lau, KS Law.
Critical revision for important intellectual content: BYH Ng, ELM Yu, KS Law, ACK Cheng.
Conflicts of interest
All authors have disclosed no conflict of interest.
Declaration
The initial abstract was presented at the “ESTRO
meets Asia” Conference 2019, Singapore, 6-8 December 2019.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sector.
Ethics approval
This study was approved by the Kowloon West Cluster
research ethics committee (Ref KW/EX-19-020(131-08)) and the requirement
for patient consent was waived by the committee.
References
1. Bolla M, Collette L, Blank L, et al.
Long-term results with immediate androgen suppression and external
irradiation in patients with locally advanced prostate cancer (an EORTC
study): a phase III randomised trial. Lancet 2002;360:103-6. Crossref
2. Pilepich MV, Winter K, Lawton C, et al.
Androgen suppression adjuvant to definitive radiotherapy in prostate
carcinoma—long-term results of phase III RTOG 85-31. Int J Radiat Oncol
Biol Phys 2005;61:1285-90. Crossref
3. Michalski J, Moughan J, Purdy J, et al.
Effect of standard vs dose-escalated radiation therapy for patients with
intermediate-risk prostate cancer: The NRG Oncology RTOG 0126 randomized
clinical trial. JAMA Oncol 2018;4:e180039. Crossref
4. Dearnaley D, Syndikus I, Mossop H, et
al. Conventional versus hypofractionated high-dose intensity-modulated
radiotherapy for prostate cancer: 5-year outcomes of the randomized, non
inferiority, phase 3 CHHiP trial. Lancet Oncol 2016;17:1047-60. Crossref
5. Aluwini S, Pos F, Schimmel E, et al.
Hypofractionated versus conventionally fractionated radiotherapy for
patients with prostate cancer (HYPRO): acute toxicity results from a
randomised non-inferiority phase 3 trial. Lancet Oncol 2015;16:274-83. Crossref
6. Zelefsky MJ, Levin EJ, Hunt M, et al.
Incidence of late rectal and urinary toxicities after three-dimensional
conformal radiotherapy and intensity-modulated radiotherapy for localized
prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:1124-9. Crossref
7. Heemsbergen WD, Peeters ST, Koper PC,
Hoogeman MS, Lebesque JV. Acute and late gastrointestinal toxicity after
radiotherapy in prostate cancer patients: consequential late damage. Int J
Radiat Oncol Biol Phys 2006;66:3-10. Crossref
8. Poon DM, Chan SL, Leung CM, et al.
Efficacy and toxicity of intensity-modulated radiation therapy for
prostate cancer in Chinese patient. Hong Kong Med J 2013;19:407-15. Crossref
9. Delobel J, Gnep K, Ospina JD, et al.
Nomogram to predict rectal toxicity following prostate cancer
radiotherapy. PLoS One 2017;12:e0179845. Crossref
10. Feng M, Hanlon AL, Pisansky T, et al.
Predictive factors for late genitourinary and gastrointestinal toxicity in
patients with prostate cancer treated with adjuvant or salvage
radiotherapy. Int J Radiat Oncol Biol Phys 2007;68:1417-23. Crossref
11. Fellin G, Fiorino C, Rancati T, et al.
Clinical and dosimetric predictors of late rectal toxicity after conformal
radiation for localized prostate cancer: results of a large multicenter
observational study. Radiother Oncol 2009;93:197-202. Crossref
12. Fuentes-Raspall R, Inoriza J,
Rosello-Serrano A, Auñón- Sanz C, Garcia-Martin P, Oliu-Isern G. Late
rectal and bladder toxicity following radiation therapy for prostate
cancer: predictive factors and treatment results. Rep Pract Oncol
Radiother 2013;18:298-303. Crossref
13. Takeda K, Ogawa Y, Ariga H, et al.
Clinical correlations between treatment with anticoagulants/antiaggregants
and late toxicity after radiotherapy for prostate cancer. Anticancer Res
2009;29:1831-4.
14. Sanguineti G, Agostinelli S, Foppiano
S, et al. Adjuvant androgen deprivation impacts late rectal toxicity after
conformal radiotherapy of prostate carcinoma. Br J Cancer 2002;86:1743-7.
Crossref
15. Fuentes-Raspall R, Inoriza J,
Martí-Utzet MJ, Auñón-Sanz C, Garcia-Martin P, Oliu-Isern G. Hyperbaric
oxygen therapy for late rectal and bladder toxicity after radiation in
prostate cancer patients. A symptom control and qualityof- life study.
Clin Oncol (R Coll Radiol) 2012;24:e126. Crossref
16. Jones K, Evans A, Bristow R, Levin W.
Treatment of radiation proctitis with hyperbaric oxygen. Radiother Oncol
2006;78:91-4. Crossref
17. Kochhar R, Sriram PV, Sharma SC, Goel
RC, Patel F. Natural history of late radiation proctosigmoiditis treated
with topical sucralfate suspension. Dig Dis Sci 1999;44:973-8. Crossref
18. Talley NA, Chen F, King D, Jones M,
Talley NJ. Shortchain fatty acids in the treatment of radiation proctitis:
a randomized double-blind, placebo-controlled, cross-over pilot trial. Dis
Colon Rectum 1997;40:1046-50. Crossref
19. Denton A, Andreyev H, Forbes A, Maher
EJ. Systematic review for non-surgical interventions for the management of
late radiation proctitis. Br J Cancer 2002;87:134-43. Crossref
20. American Joint Committee on Cancer,
Prostate Cancer Staging, 7th edition. Available from:
https://cancerstaging.org/references-tools/quickreferences/Documents/ProstateSmall.pdf.
Accessed 1 Apr 2019.
21. US Department of Health and Human
Services, National Institutes of Health, National Cancer Institute, US
Government. Common Terminology Criteria for Adverse Events (CTCAE) version
4.03. 2010. Available from:
https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40.
Accessed 1 Apr 2019.
22. Abramowitz M, Li T, Buyyounouski MK,
et al. The Phoenix definition of biochemical failure predicts for overall
survival in patients with prostate cancer. Cancer 2008;112:55-60. Crossref
23. Bansai N, Soni A, Kaur P, Chauhan AK.
Exploring the management of radiation proctitis in current clinical
practice. J Clin Diagn Res 2016;10:XE01-XE06. Crossref
24. Skwarchuk MW, Jackson A, Zelefsky MJ,
et al. Late rectal toxicity after conformal radiotherapy of prostate
cancer (I): multivariate analysis and dose-response. Int J Radiat Oncol
Biol Phys 2000;47:103-13. Crossref
25. Herold DM, Hanlon AL, Hanks GE.
Diabetes mellitus: a predictor for late radiation morbidity. Int J Radiat
Oncol Biol Phys 1999;43:475-9. Crossref
26. Miralbell R, Taussky D, Rinaldi O, et
al. Influence of rectal volume changes during radiotherapy for prostate
cancer: a predictive model for mild-to-moderate late rectal toxicity. Int
J Radiat Oncol Biol Phys 2003;57:1280-4. Crossref