Abstract
Background
CPS + EG staging, which incorporates estrogen receptor (ER) status and tumor grade with pretreatment clinical stage (CS) and post-treatment pathologic stage (PS), has been reported to have better correlation with outcome than classic TNM staging for patients treated with neoadjuvant chemotherapy (NAC). Our goal was to evaluate the performance of CPS + EG staging system in an external cohort treated with NAC.
Methods
We reviewed patients with stages I–IIIC breast cancer treated with NAC and surgery at our institution between 1988 and 2014. ER status, Nottingham grade, treatment, American Joint Committee on Cancer (AJCC) CS before NAC and PS after NAC, and follow-up data were collected. The discrimination of CPS + EG and pathologic AJCC stage were assessed using area under the curve (AUC) for survival data.
Results
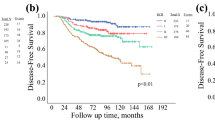
A total of 769 patients were analyzed with a median follow-up of 2.6 (range 0.0–19.4) years; 103 patients died of breast cancer. Overall, the 5-year breast cancer cause-specific survival was 81.5 % [95 % confidence interval (CI) 77.6–85.5]. The 5-year, cause-specific survival by CPS + EG score was 93.8 % score 0, 89.9 % score 1, 90.7 % score 2, 84.8 % score 3, 67.7 % score 4, and 43.4 % score 5/6. CPS + EG score was significantly associated with cause-specific survival (p < 0.001) with an AUC of 0.69 (95 % CI 0.62–0.77) at 5 years. This was higher than the AUC of 0.63 (95 % CI 0.56–0.70) for AJCC PS (p = 0.10).
Conclusions
This study validates the CPS + EG staging system using Nottingham grade in an external cohort. Addition of tumor biology and treatment response shows promise in improving survival estimates for patients treated with NAC.



Similar content being viewed by others
References
Mauri D, Pavlidis N, Ioannidis JPA. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–94.
Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local–regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15(7):2483–93.
Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–85.
Boughey J, Peintinger F, Meric-Bernstam F, et al. Impact of preoperative versus postoperative chemotherapy on the extent and number of surgical procedures in patients treated in randomized clinical trials for breast cancer. Ann Surg. 2006;244(3):464–70.
Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17(2):460–9.
Von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–804.
Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414–22.
Harigopal M, Barlow WE, Tedeschi G, et al. Multiplexed assessment of the Southwest Oncology Group-directed Intergroup Breast Cancer Trial S9313 by AQUA shows that both high and low levels of HER2 are associated with poor outcome. Am J Pathol. 2010;176(4):1639–47.
Ross JS. Multigene classifiers, prognostic factors, and predictors of breast cancer clinical outcome. Adv Anat Pathol. 2009;16(4):204–15.
Yi M, Mittendorf E, Cormier JN, et al. Novel staging system for predicting disease-specific survival in patients with breast cancer treated with surgery as the first intervention: time to modify the current American Joint Committee on Cancer staging system. J Clin Oncol. 2011;29(35):4654–61.
Mittendorf EA, Jeruss JS, Tucker SL, et al. Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol. 2011;29(15):1956–62.
Marmé F, Aigner J, Lorenzo Bermejo J, Sinn P, Sohn C, Jager D, Schneeweiss A. Neoadjuvant epirubicin, gemcitabine and docetaxel for primary breast cancer: long-term survival data and major prognostic factors based on two consecutive neoadjuvant phase I/II trials. Int J Cancer 2013;133(4):1006–15.
Marmé F, Lederer B, Blohmer J, et al. Utility of the CPS + EG staging system in hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer treated with neoadjuvant chemotherapy. Eur J Cancer 2016;53:65–74.
A study of palbociclib in addition to standard endocrine treatment in hormone receptor positive Her2 normal patients with residual disease after neoadjuvant chemotherapy and surgery (PENELOPE-B). https://clinicaltrials.gov/ct2/show/NCT01864746. Accessed 28 March 2016.
Olaparib as adjuvant treatment in patients with germline BRCA mutated high risk HER2 negative primary breast cancer (OlympiA). https://clinicaltrials.gov/ct2/show/NCT02032823. Accessed 28 March 2016.
Dalton LW, Page DL, Dupont WD. Histologic grading of breast carcinoma: a reproducibility study. Cancer 1994;73(11):2765–70.
Carey LA, Metzger R, Dees EC, et al. American Joint Committee on Cancer tumor-node-metastasis stage after neoadjuvant chemotherapy and breast cancer outcome. J Natl Cancer Inst. 2005;97(15):1137–42.
Simpson JF, Gray R, Dressler LG, et al. Prognostic value of histologic grade and proliferative activity in axillary node-positive breast cancer: results from the Eastern Cooperative Oncology Group Companion Study, EST 4189. J Clin Oncol. 2000;18(10):2059–69.
Blanche P, Dartigues JF, Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med. 2013;32(30):5381–97.
Teshome M, Tucker S, Hunt K, Mittendorf E. Prognostic value of combined clinical and pathologic staging variables in predicting local–regional recurrence following neo-adjuvant chemotherapy for breast cancer. Ann Surg Oncol. 2015;22(Supplement 1):S15.
AJCC (American Joint Committee on Cancer). Breast cancer staging. In: Cancer staging manual, 7th ed. New York: Springer; 2010.
Park YH, Lee SJ, Cho EY, et al. Clinical relevance of TNM staging system according to breast cancer subtypes. Ann Oncol. 2011;22(7):1554–60.
Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28:1829–34.
Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–34.
Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet sOncol. 2010;11(1):55–65.
Rakha EA, Reis-Filho JS, Baehner F, et al. Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res. 2010;12(4):207.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19(5):403-10.
Black M, Opler S, Speer F. Survival in breast cancer cases in relation to the structure of the primary tumor and regional lymph nodes. Surg Gynecol Obstet. 1955;100(5):543-51.
Mittendorf E, Vila J, Tucker S, et al. The Neo-Bioscore update for staging breast cancer treated with neoadjuvant chemotherapy: Incorporation of prognostic biologic factors into staging after treatment. JAMA Oncol. 2016. doi:10.1001/jamaoncol.2015.6478.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. Jad M. Abdelsattar and Dr. Zahraa Al-Hilli are joint first authors.
Rights and permissions
About this article
Cite this article
Abdelsattar, J.M., Al-Hilli, Z., Hoskin, T.L. et al. Validation of the CPS + EG Staging System for Disease-Specific Survival in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Ann Surg Oncol 23, 3206–3211 (2016). https://doi.org/10.1245/s10434-016-5324-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5324-y