Abstract
Background
The extracellular matrix metalloproteases MMP-9 and MMP-2 are critical for the invasive potential of tumors. However, it is not clear which of the two plays the predominant role in tumor invasion and progression. In the present study, we compared the clinical efficacy of MMP-9 and MMP-2 overexpression for predicting tumor recurrence and survival after surgical resection in HCC patients.
Materials and Methods
MMP-9 and MMP-2 expression in HCC cell lines and in vitro HCC invasion model were detected by quantitative RT-PCR and immunofluorescence. The expression levels of MMP-9 and MMP-2 were assessed by immunohistochemistry in HCC tissue microarrays from HCC patients (study set) who underwent curative resection. The clinicopathological data were retrospectively analyzed. The results were further verified in an independent cohort of 92 HCC patients (validation set).
Results
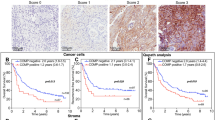
Univariate analysis demonstrated that high expression of MMP-9 was associated with both time to recurrence (TTR, P = .015) and overall survival (OS, P = .024), whereas high expression of MMP-2 was only correlated with TTR (P = .041). Multivariate analysis confirmed that MMP-9 expression was an independent predictor of TTR and OS. The coindex of MMP-9 and preoperative serum AFP levels was significantly correlated with TTR and OS (P = .036 and P = .040), but the coindex of MMP-2 and AFP did not show prognostic significance for either TTR or OS (P = .067 and P = .053). The prognostic value of MMP-9 overexpression was validated in the independent data set.
Conclusion
MMP-9 is superior to MMP-2 for the prediction of tumor recurrence and survival in HCC patients after surgical resection.



Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76.
Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet. 2009;373:614–6.
Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH, et al. A decade’s studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–96.
Hao K, Luk JM, Lee NP, Mao M, Zhang C, Ferguson MD, et al. Predicting prognosis in hepatocellular carcinoma after curative surgery with common clinicopathologic parameters. BMC Cancer. 2009;9:389.
Qin LX, Tang ZY. Recent progress in predictive biomarkers for metastatic recurrence of human hepatocellular carcinoma: a review of the literature. J Cancer Res Clin Oncol. 2004;130:497–513.
Sakamoto Y, Mafune K, Mori M, Shiraishi T, Imamura H, Mori M, et al. Overexpression of MMP-9 correlates with growth of small hepatocellular carcinoma. Int J Oncol. 2000;17:237–43.
Jezierska A, Motyl T. Matrix metalloproteinase-2 involvement in breast cancer progression: a mini-review. Med Sci Monit. 15;2009:RA32–40.
Zhang Q, Chen X, Zhou J, Zhang L, Zhao Q, Chen G, et al. CD147, MMP-2, MMP-9 and MVD-CD34 are significant predictors of recurrence after liver transplantation in hepatocellular carcinoma patients. Cancer Biol Ther. 2006;5:808–14.
Bagnoli F, Oliveira VM, Silva MA, Taromaru GC, Rinaldi JF, Aoki T. The interaction between aromatase, metalloproteinase 2,9 and cd44 in breast cancer. Rev Assoc Med Bras. 2010;56:472–7.
Yeh HC, Lin SM, Chen MF, Pan TL, Wang PW, Yeh CT. Evaluation of serum matrix metalloproteinase (MMP)-9 to MMP-2 ratio as a biomarker in hepatocellular carcinoma. Hepatogastroenterology. 2010;57:98–102.
Giannelli G, Bergamini C, Marinosci F, Fransvea E, Quaranta M, Lupo L, et al. Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular carcinoma. Int J Cancer. 2002;97:425–31.
Chen JS, Wang Q, Fu XH, Huang XH, Chen XL, Cao LQ, et al. Involvement of PI3 K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma: association with MMP-9. Hepatol Res. 2009;39:177–86.
Hah N, Lee ST. An absolute role of the PKC-dependent NF-kappaB activation for induction of MMP-9 in hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2003;305:428–33.
Arii S, Mise M, Harada T, Furutani M, Ishigami S, Niwano M, et al. Overexpression of matrix metalloproteinase 9 gene in hepatocellular carcinoma with invasive potential. Hepatology. 1996;24:316–22.
Zhao P, Zhang W, Tang J, Ma XK, Dai JY, Li Y, et al. Annexin II promotes invasion and migration of human hepatocellular carcinoma cells in vitro via its interaction with HAb18G/CD147. Cancer Sci. 2010;101:387–95.
Kohga K, Tatsumi T, Takehara T, Tsunematsu H, Shimizu S, Yamamoto M, et al. Expression of CD133 confers malignant potential by regulating metalloproteinases in human hepatocellular carcinoma. J Hepatol. 2010;52:872–9.
Miyoshi A, Kitajima Y, Sumi K, Sato K, Hagiwara A, Koga Y, et al. Snail and SIP1 increase cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. Br J Cancer. 2004;90:1265–73.
Torimura T, Ueno T, Kin M, Harada R, Nakamura T, Kawaguchi T, et al. Autocrine motility factor enhances hepatoma cell invasion across the basement membrane through activation of beta1 integrins. Hepatology. 2001;34:62–71.
Tang J, Cui J, Chen R, Guo K, Kang X, Li Y, et al. A three-dimensional cell biology model of human hepatocellular carcinoma in vitro. Tumour Biol. 2011;32:469–79.
Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–9.
Morimoto O, Nagano H, Sakon M, Fujiwara Y, Yamada T, Nakagawa H, et al. Diagnosis of intrahepatic metastasis and multicentric carcinogenesis by microsatellite loss of heterozygosity in patients with multiple and recurrent hepatocellular carcinomas. J Hepatol. 2003;39:215–21.
Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–9.
Johansson N, Ahonen M, Kahari VM. Matrix metalloproteinases in tumor invasion. Cell Mol Life Sci. 2000;57:5–15.
Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67.
Vihinen P, Ala-aho R, Kahari VM. Matrix metalloproteinases as therapeutic targets in cancer. Curr Cancer Drug Targets. 2005;5:203–20.
Vihinen P, Kahari VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer. 2002;99:157–66.
Yang P, Yuan W, He J, Wang J, Yu L, Jin X, et al. Overexpression of EphA2, MMP-9, and MVD-CD34 in hepatocellular carcinoma: Implications for tumor progression and prognosis. Hepatol Res. 2009;39:1169–77.
Nart D, Yaman B, Yilmaz F, Zeytunlu M, Karasu Z, Kiliç M. Expression of matrix metalloproteinase-9 in predicting prognosis of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2010;16:621–30.
Chung TW, Lee YC, Kim CH. Hepatitis B viral HBx induces matrix metalloproteinase-9 gene expression through activation of ERK and PI-3K/AKT pathways: involvement of invasive potential. FASEB J. 2004;18:1123–5.
Maatta M, Soini Y, Liakka A, Autio-Harmainen H. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9, and membrane type 1-MMP in hepatocellular and pancreatic adenocarcinoma: implications for tumor progression and clinical prognosis. Clin Cancer Res. 2000;6:2726–34.
Watari M, Watari H, DiSanto ME, Chacko S, Shi GP, Strauss JF III. Pro-inflammatory cytokines induce expression of matrix-metabolizing enzymes in human cervical smooth muscle cells. Am J Pathol. 1999;154:1755–62.
Sawada S, Murakami K, Murata J, Tsukada K, Saiki I. Accumulation of extracellular matrix in the liver induces high metastatic potential of hepatocellular carcinoma to the lung. Int J Oncol. 2001;19:65–70.
Kim JR, Kim CH. Association of a high activity of matrix metalloproteinase-9 to low levels of tissue inhibitors of metalloproteinase-1 and -3 in human hepatitis B-viral hepatoma cells. Int J Biochem Cell Biol. 2004;36:2293–306.
Chung TW, Moon SK, Lee YC, Kim JG, Ko JH, Kim CH. Enhanced expression of matrix metalloproteinase-9 by hepatitis B virus infection in liver cells. Arch Biochem Biophys. 2002;408:147–54.
Ye QH, Qin LX, Forgues M, He P, Kim JW, Peng AC, et al. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9:416–23.
Takafuji V, Forgues M, Unsworth E, Goldsmith P, Wang XW. An osteopontin fragment is essential for tumor cell invasion in hepatocellular carcinoma. Oncogene. 2007;26:6361–71.
Ji XN, Ye SL, Li Y, Tian B, Chen J, Gao DM, et al. Contributions of lung tissue extracts to invasion and migration of human hepatocellular carcinoma cells with various metastatic potentials. J Cancer Res Clin Oncol. 2003;129:556–64.
Hayasaka A, Suzuki N, Fujimoto N, Iwama S, Fukuyama E, Kanda Y, et al. Elevated plasma levels of matrix metalloproteinase-9 (92-kd type IV collagenase/gelatinase B) in hepatocellular carcinoma. Hepatology. 1996;24:1058–62.
Wu KJ, Zeng J, Zhu GD, Zhang LL, Zhang D, Li L, et al. Silibinin inhibits prostate cancer invasion, motility and migration by suppressing vimentin and MMP-2 expression. Acta Pharmacol Sin. 2009;30:1162–8.
Kenny HA, Lengyel E. MMP-2 functions as an early response protein in ovarian cancer metastasis. Cell Cycle. 2009;8:683–8.
Acknowledgment
The authors sincerely thank Professors Jia Fan, Jian Zhou, Shuang-Jian Qiu, and Zhi-Quan Wu for their help in collecting human HCC tissue samples. This study was sponsored by grants from National Natural Science Foundation of China (No. 30772062, No. 81071902, and No. 81000909), China National High-Tech Research and Development Program (2006AA02A-308), China National Key Projects for Infectious Disease (2008ZX10002-021 and 2008ZX 10002-017), Shanghai Pujiang Program (No.08PJ140300), and Shanghai Natural Science Foundation (09ZR1406400).
Author information
Authors and Affiliations
Corresponding author
Additional information
Rongxin Chen and Jiefeng Cui contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, R., Cui, J., Xu, C. et al. The Significance of MMP-9 Over MMP-2 in HCC Invasiveness and Recurrence of Hepatocellular Carcinoma After Curative Resection. Ann Surg Oncol 19 (Suppl 3), 375–384 (2012). https://doi.org/10.1245/s10434-011-1836-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-011-1836-7