Abstract
Background
Previous studies revealed serosal invasion as one of the most important predictors of peritoneal micrometastasis. However, even for cancers with serosal invasion, the macroscopic serosal appearance is highly heterogeneous. The aim of the present study was to propose a macroscopic serosal classification (MSC) and to investigate the validity of this classification as a predictor of peritoneal recurrence.
Materials and Methods
Clinicopathologic features including MSC of 1528 patients with pT3/pT4a stage gastric cancers who underwent potentially radical surgery were retrospectively reviewed. MSC was classified as reactive type, nodular type, tendonoid type, and color-diffused type according to the macroscopic serosal appearance.
Results
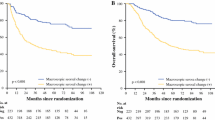
There were significant differences in tumor size, location, Bormann type, Lauren grade, lymphatic and/or blood vessels invasion (LBVI), width of serosa changes, depth of invasion, number of nodes metastasis, lymph node metastasis ratio, pN stage, and peritoneal cytology between patients with different types of serosa. Multivariate analysis revealed MSC, as well as depth of invasion, Lauren grade, and pN stage, significantly predicted the presence of peritoneal-free cancer cells. Both MSC and peritoneal cytology significantly correlated with patient survival. However, only MSC significantly predicted peritoneal recurrence on multivariate analysis, but peritoneal cytology did not, indicating MSC was more sensitive than cytologic examination. Further investigation suggested MSC and pN stage were also independent predictors of peritoneal recurrence for patients with negative peritoneal cytology.
Conclusions
The MSC sensitively predicts the presence of peritoneal micrometastasis for pT3/pT4a-stage gastric cancer patients who underwent potentially radical surgery. Consequently, it might be considered a good indicator to guide perioperative adjuvant therapy for patients with high risk of peritoneal recurrence.


Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96.
Goggins WB, Wong GK. Poor survival for US Pacific Islander cancer patients: evidence from the Surveillance, Epidemiology, and End Results database: 1991 to 2004. J Clin Oncol. 2007;25:5738–41.
D’Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808–16.
Roviello F, Marrelli D, de Manzoni G, Morgagni P, Di Leo A, Saragoni L, et al. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg. 2003;90:1113–9.
Benevolo M, Mottolese M, Cosimelli M, Tedesco M, Giannarelli D, Vasselli S, et al. Diagnostic and prognostic value of peritoneal immunocytology in gastric cancer. J Clin Oncol. 1998;16:3406–11.
Bonenkamp JJ, Songun I, Hermans J, van de Velde CJ. Prognostic value of positive cytology findings from abdominal washings in patients with gastric cancer. Br J Surg. 1996;83:672–4.
Abe S, Yoshimura H, Tabata H, Tachibana M, Monden N, Nakamura T, et al. Curative resection of gastric cancer: limitation of peritoneal lavage cytology in predicting the outcome. J Surg Oncol. 1995;59:226–9.
Kodera Y, Nakanishi H, Ito S, Yamamura Y, Kanemitsu Y, Shimizu Y, et al. Quantitative detection of disseminated free cancer cells in peritoneal washes with real-time reverse transcriptase-polymerase chain reaction: a sensitive predictor of outcome for patients with gastric carcinoma. Ann Surg. 2002;235:499–506.
Nakanishi H, Kodera Y, Yamamura Y, Ito S, Kato T, Ezaki T, et al. Rapid quantitative detection of carcinoembryonic antigen-expressing free tumor cells in the peritoneal cavity of gastric cancer patients with real-time RT-PCR on the lightcycler. Int J Cancer. 2000;89:411–7.
Ito S, Nakanishi H, Kodera Y, Mochizuki Y, Tatematsu M, Yamamura Y. Prospective validation of quantitative CEA mRNA detection in peritoneal washes in gastric carcinoma patients. Br J Cancer. 2005;93:986–92.
Baba H, Korenaga D, Okamura T, Saito A, Sugimachi K. Prognostic factors in gastric cancer with serosal invasion: univariate and multivariate analyses. Arch Surg. 1989;124:1061–4.
Sobin L, Gospodarowicz M, Wittekind C, eds. TNM Classification of Malignant Tumours. 7th ed. International Union Against Cancer (UICC). New York: Wiley, 2009.
Ichiyoshi Y, Maehara Y, Tomisaki S, Oiwa H, Sakaguchi Y, Ohno S, et al. Macroscopic intraoperative diagnosis of serosal invasion and clinical outcome of gastric cancer: risk of underestimation. J Surg Oncol. 1995;59:255–60.
Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, et al. Peritoneal washing cytology: prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol. 1999;72:60–4 (discussion 64–5).
Japanese Gastric Cancer A. Japanese classification of gastric carcinoma—2nd English edition. Gastric Cancer. 1998;1:10–24.
Homma Y, Ushida S, Yamada M, Kobayashi H, Suzuki K. Positive peritoneal washing cytology in multiple cavities can predict poor prognosis of advanced gastric cancer patients. Ann Surg Oncol. 2010;17:455–60.
Marrelli D, Roviello F, de Manzoni G, Morgagni P, Di Leo A, Saragoni L, et al. Different patterns of recurrence in gastric cancer depending on Lauren’s histological type: longitudinal study. World J Surg. 2002;26:1160–5.
Soga K, Ichikawa D, Yasukawa S, Kubota T, Kikuchi S, Fujiwara H, et al. Prognostic impact of the width of subserosal invasion in gastric cancer invading the subserosal layer. Surgery. 2010;147:197–203.
Bando E, Kawamura T, Kinoshita K, Takahashi S, Maeda A, Osada S et al. Magnitude of serosal changes predicts peritoneal recurrence of gastric cancer. J Am Coll Surg. 2003;197:212–22.
Abe S, Shiraishi M, Nagaoka S, Yoshimura H, Dhar DK, Nakamura T. Serosal invasion as the single prognostic indicator in stage III A (T3N1M0) gastric cancer. Surgery. 1991;6:582–8.
Baba H, Korenaga D, Haraguchi M, Okamura T, Saito A, Watanabe A, et al. Width of serosal invasion and prognosis in advanced human gastric cancer with special reference to the mode of tumor invasion. Cancer. 1989;15:2482–6.
Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer. 1999;85:529–34.
Rosen HR, Jatzko G, Repse S, Potrc S, Neudorfer H, Sandbichler P, et al. Adjuvant intraperitoneal chemotherapy with carbon-adsorbed mitomycin in patients with gastric cancer: results of a randomised multi-center trial of the Austrian Working Group for Surgical Oncology. J Clin Oncol. 1998;16:2733–8.
Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–43.
Chua TC, Morris DL, Esquivel J. Impact of the peritoneal surface disease severity score on survival in patients with colorectal cancer peritoneal carcinomatosis undergoing complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17:1330–6.
Yan TD, Black D, Sugarbaker PH, Zhu J, Yonemura Y, Petrou G, et al. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol. 2007;14:2702–13.
Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27:6237–42.
Bakkevold KE, Kambestad B. Morbidity and mortality after radical and palliative pancreatic cancer surgery. Risk factors influencing the short-term results. Ann Surg. 1993;217:356–68.
Kusamura S, Younan R, Baratti D, Costanzo P, Favaro M, Gavazzi C, et al. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion: analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen technique. Cancer. 2006;106:1144–53.
Elias D, Goere D, Blot F, Billard V, Pocard M, Kohneh-Shahri N, et al. Optimization of hyperthermic intraperitoneal chemotherapy with oxaliplatin plus irinotecan at 43 degrees C after compete cytoreductive surgery: mortality and morbidity in 106 consecutive patients. Ann Surg Oncol. 2007;14:1818–24.
Yan TD, Black D, Savady R, Sugarbaker PH. Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol. 2006;24:4011–9.
Chua TC, Yan TD, Saxena A, Morris DL. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: a systematic review of morbidity and mortality. Ann Surg. 2009;249:900–7.
Acknowledgment
Supported by grants from the National Natural Science Foundation of China (no. 30901419).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sun, Z., Xu, Yy., Wang, Zn. et al. Macroscopic Serosal Classification Predicts Peritoneal Recurrence for Patients with Gastric Cancer Underwent Potentially Curative Surgery. Ann Surg Oncol 18, 1068–1080 (2011). https://doi.org/10.1245/s10434-010-1449-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-010-1449-6