Abstract
Background
Surgical cytoreduction combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has been recently advocated as the standard of care for pseudomyxoma peritonei (PMP). We reviewed our 10-year monoinstitutional case series to identify selection factors predicting postoperative outcome.
Methods
One hundred and four patients with PMP were operated on with the aim of performing adequate cytoreduction (residual tumor nodules ≤2.5 mm) and closed-abdomen HIPEC with mytomicin-C and cisplatin. Previously, 26 patients had systemic chemotherapy. PMP was histologically classified into disseminated peritoneal adenomucinosis (DPAM), peritoneal mucinous carcinomatosis (PMCA), and intermediate/discordant group (ID). Immunohistochemical stains were performed for cytokeratin (CK)-7, CK-20, CDX-2, MUC-2, MUC-5AC, CD-44s. The significance of 22 potential clinical, pathological, and biological prognostic variables was assessed by multivariate analysis.
Results
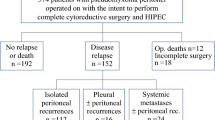
Adequate cytoreduction was performed in 89 patients, suboptimal cytoreduction in six, palliative surgery in nine. Operative mortality was 1%. Seventy-eight patients were diagnosed with DPAM, 26 with PMCA, and none with ID. Median follow-up was 37 months (range, 1–110) for the overall series. Five-year overall survival (OS) and progression-free survival (PFS) were 78.3% and 31.1%, respectively. At multivariate analysis, adequate cytoreduction, no previous systemic chemotherapy, and DPAM correlated to better OS and PFS, elevated serum CA19.9 correlated only to better PFS. In most cases, CK20, CDX-2, and MUC-2 were diffusely positive, while CK-7, MUC-5AC, and CD44s were variably expressed. CK20 expression correlated to prognosis at univariate analysis.
Conclusions
Favorable outcome after comprehensive treatment can be expected in patients with DPAM, not treated with preoperative systemic chemotherapy and amenable to adequate cytoreduction. MUC-2, CK-20, and CD44s expression may be related to PMP unique biologic behavior.


Similar content being viewed by others
References
Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? The Lancet Oncol 2006; 7:69–76
Brendan MJ, Cecil TD. The etiology, clinical presentation and management of pseudomyxoma peritonei. Surg Oncol Clin N Am 2003; 12:585–603
Ronnett BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis: a clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis and relationship to “pseudomyxoma peritonei.” Am J Surg Pathol 1995; 19:1390–408
Szych C, Staebler A, Connolly DC, Wu R, Cho KR, Ronnett BM. Molecular genetic evidence supporting the clonality and appendiceal origin of pseudomyxoma peritonei in women. Am J Pathol 1999; 154:1849–55
Carr NJ, Emory TS, Sobin LH. Epithelial neoplasms of the appendix and colorectum: an analysis of cell proliferation, apaptosis and expression of p53, CD44, bcl-2. Arch Pathol Lab Med 2002; 126:837–41
Gough DB, Donohue JH, Schutt AJ, et al. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg 1994; 219:112–9
Miner TJ, Shia J, Jaques DP, Klimstra DS, Brennan MF, Coit DG. Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg 2005; 241:300–8
Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol 2003; 27:1089–103
Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995; 221:29–42
Sugarbaker PH. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol 1999; 6:727–31
Deraco M, Baratti D, Inglese MG, et al. Peritonectomy and intra-peritoneal hyperthermic perfusion: a strategy that has confirmed its efficacy in patients with pseudomyxoma peritonei. Ann Surg Oncol 2004; 11:393–8
Gonzalez-Moreno S, Sugarbaker PH. Right hemicolectomy does not confer a survival advantage in patients with mucinous carcinoma of the appendix and peritoneal seeding. Br J Surg 2004; 91:304–11
Yan TD, Links M, Xu ZY, Kam PC, Glenn D, Morris DL. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei from appendiceal mucinous neoplasms. Br J Surg 2006; 93:1270–6
Stewart JH, Shen P, Russell GB, et al. Appendiceal neoplasms with peritoneal dissemination: outcomes after cytoreductive surgery and intraperitoneal hyperthermic chemotherapy. Ann Surg Oncol 2006; 13:624–34
Smeenk RM, Verwaal VJ, Antonini N, Zoetmulder FAN. Survival analysis of pseudomyxoma peritonei patients treated by cytoreductive surgery and hyperthermic chemotherapy. Ann Surg 2007; 245:104–9
Murphy EM, Sexton R, Moran BJ. Early results of surgery in 123 patients with pseudomyxoma peritonei from a perforated appendiceal neoplasm. Dis Colon Rectum 2006; 50:37–42
Nonaka D, Kusamura S, Baratti D, Casali P, Younan R, Deraco M. CDX-2 expression in pseudomyxoma peritonei: a clinicopathological study of 42 cases. Histopathology 2006; 49:381–7
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and Response Criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5:649–55
Jacquet P, Jelinek JS, Chang D, Koslowe P, Sugarbaker PH. Abdominal computed tomographic scan in the selection of patients with mucinous peritoneal carcinomatosis for cytoreductive surgery. J Am Coll Surg 1995; 181:530–8
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92:205–16
Esquivel JE, Sugarbaker PH. Elective surgery in recurrent colon cancer with peritoneal seeding: when to and when not to. Cancer Ther 1998; 1:321–5
Jaquet P, Sugarbaker PH. Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J Exp Clin Cancer Res 1996; 15:49–58
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Soc 1958; 53:457–81
Cox DR. Regression models and life tables (with discussion). J R Stat Soc B 1972; 34:187–220
Los G, Mutsaers PH, Lenglet WJ, Baldew GS, McVie JG. Platinum distribution in intraperitoneal tumors after intraperitoneal cisplatin treatment. Cancer Chemother Pharmacol 1990; 25:389–94
Bradley RF, Stewart JH, Russell GB, Levine EA, Geisinger KR. Pseudomyxoma peritonei of appendiceal origin: a clinicopathological analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol 2006; 30:551–9
Baratti D Kusamura S, Martinetti A, Seregni E, Laterza B, Oliva GD, Deraco M. Prognostic Value of Circulating Tumor Markers in Patients with Pseudomyxoma Peritonei Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol 2007; 14:2300–8
O’Connell JT, Tomlinson JS, Roberts AA McGonigle KF, Sanford HB. Pseudomyxoma peritonei is a disease of MUC2-expressing goblet cells. Am J Pathol 2002; 161:551–64
Maheshwari V, Tsung A, Lin Y, Zeh HJ III, Finkelstein SD, Bartlett DL. Analysis of loss of heterozygosity for tumor-suppressor genes can accurately classify and predict the clinical behavior of mucinous tumors arising from the appendix. Ann Surg Oncol 2006; 13:1610–6
Bibi R, Pranesh N, Saunders MP, Wilson MS, O’Dwyer ST, Stern PL, Renehan AG. A specific cadherin phenotype may characterise the disseminating yet non-metastatic behaviour of pseudomyxoma peritonei. Br J Cancer 2006; 95:1258–64
Park SY, Kim HS, Hong EK, Kim WH. Expression of cytokeratins 7 and 20 in primary carcinomas of the stomach and colorectum and their value in the differential diagnosis of metastatic carcinomas to the ovary. Hum Pathol 2002; 33:1078–85
Drummond F, Putt W, Fox M, Edwards YH. Cloning and chromosome assignment of the human CDX2 gene. Ann Hum Genet 1997; 61:393–400
Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol 2003; 27:303–10
Vang R, Gown AM, Zhao C, Barry TS, Isacson C, Richardson MS, Ronnett BM. Ovarian mucinous tumors associated with mature cystic teratomas: morphologic and immunohistochemical analysis identifies a subset of potential teratomatous origin that shares features of lower gastrointestinal tract mucinous tumors more commonly encountered as secondary tumors in the ovary. Am J Surg Pathol 2007; 31:854–69
Isozaki H, Ohyama T, Mabuchi H. Expression of cell adhesion molecule CD44 and syalil-Lewis A in gastric carcinoma and colorectal carcinoma in association with hepatic metastasis. Int J Oncol 1998; 13:935–42
Velchich A, Yang W,Heyer J, et al. Colorectal cancer in mice genetically deficient in the mucin MUC2. Science 2002; 295:1726–9
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baratti, D., Kusamura, S., Nonaka, D. et al. Pseudomyxoma Peritonei: Clinical Pathological and Biological Prognostic Factors in Patients Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Ann Surg Oncol 15, 526–534 (2008). https://doi.org/10.1245/s10434-007-9691-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-007-9691-2