Abstract
Background
Preoperative combined-modality therapy (CMT) is the preferred treatment for locally advanced rectal cancer (endorectal ultrasonography [ERUS] T3–4, N1, or clinically bulky) and achieves a pathologic complete response (pCR) in 4% to 33% of patients. However, the prognostic significance of pCR remains unclear.
Methods
A prospectively collected database was queried to identify 200 patients with locally advanced disease treated from 1992 to 2002. The pCR group was defined as having no evidence of viable tumor on pathologic analysis. The no-downstaging group was defined as no difference between the pre-CMT ERUS stage and the pathologic stage. Those achieving some downstaging but not pCR were excluded. Patients were treated with CMT (5040 cGy of radiation and 5-fluorouracil–based chemotherapy) followed by surgery, and 51 (85%) in the pCR group and 129 (92%) in the no-downstaging group (P = .1) received postoperative chemotherapy. Recurrence-free survival (RFS) and overall survival (OS) were determined by using the Kaplan-Meier method.
Results
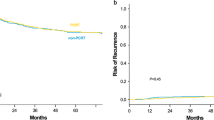
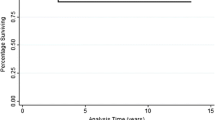
The median follow-up was 38.6 months (range, 18.2–124.9 months). The pCR (n = 60) and control (n = 140) groups were similar in age (P = .6), sex (P = .4), distance of the tumor from the anal verge (P = .3), pre-CMT ERUS stage (P = .2), and comorbidities (P = .2). The 5-year RFS was 96% and 54% in the pCR and control groups, respectively (P < .00001); the 5-year OS was 90% and 68% (P = .009). Sphincter-preservation rates were higher in the pCR group (P = .01).
Conclusions
Rectal cancer patients with pCR after preoperative CMT have improved RFS, OS, and sphincter preservation compared with patients without downstaging. Because pCR seems to be associated with better outcome, an understanding of the factors governing the response to CMT should be pursued.


Similar content being viewed by others
References
Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351:1731–40
Ahmad NR, Nagle D. Long-term results of preoperative radiation therapy alone for stage T3 and T4 rectal cancer. Br J Surg 1997; 84:1445–8
Mehta VK, Poen J, Ford J, et al. Radiotherapy, concomitant protracted-venous-infusion 5-fluorouracil, and surgery for ultrasound-staged T3 or T4 rectal cancer. Dis Colon Rectum 2001; 44:52–8
Guillem JG, Chessin DB, Cohen AM, et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg 2005; 241:829–36
Garcia-Aguilar J, Hernandez de Anda E, Sirivongs P, Lee SH, Madoff RD, Rothenberger DA. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum 2003; 46:298–304
Janjan NA, Abbruzzese J, Pazdur R, et al. Prognostic implications of response to preoperative infusional chemoradiation in locally advanced rectal cancer. Radiother Oncol 1999; 51:153–60
Onaitis MW, Noone RBF, R., Hurwitz H, et al. Complete response to neoadjuvant chemoradiation for rectal cancer does not influence survival. Ann Surg Oncol 2001; 8:801–6.
Theodoropoulos G, Wise WE, Padmanabhan A, et al. T-level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum 2002; 45:895–903
Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 2004; 240:711–7; discussion 717–8
Hildebrandt U, Feifel G, Schwarz HP, Scherr O. Endorectal ultrasound: instrumentation and clinical aspects. Int J Colorectal Dis 1986; 1:203–7
Beynon J, Roe AM, Foy DM, Channer JL, Virjee J, Mortensen NJ. Preoperative staging of local invasion in rectal cancer using endoluminal ultrasound. J R Soc Med 1987; 80:23–4
Skibber J, Hoff PM, Minsky BD. Cancer of the Rectum. Philadelphia: Lippincott Williams & Wilkins, 2001
Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg 1998; 133:894–9
Enker WE, Thaler HT, Cranor ML, Polyak T. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg 1995; 181:335–46
AJCC Cancer Staging Manual. 5th ed. Philadelphia: Lippincott-Raven, 1998
Ruo L, Tickoo S, Klimstra DS, et al. Long-term prognostic significance of extent of rectal cancer response to preoperative radiation and chemotherapy. Ann Surg 2002; 236:75–81
Guillem JG, Puig-La Calle J Jr, Akhurst T, et al. Prospective assessment of primary rectal cancer response to preoperative radiation and chemotherapy using 18-fluorodeoxyglucose positron emission tomography. Dis Colon Rectum 2000; 43:18–24
Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 2000; 343:905–14
Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004; 350:2343–51
Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004; 351:337–45
Starling N, Cunningham D. Monoclonal antibodies against vascular endothelial growth factor and epidermal growth factor receptor in advanced colorectal cancers: present and future directions. Curr Opin Oncol 2004; 16:385–90
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350:2335–42
Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 2004; 10:145–7
Moore HG, Gittleman AE, Minsky BD, et al. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum 2004; 47:279–86
Moore HG, Shia J, Klimstra DS, et al. Expression of p27 in residual rectal cancer after preoperative chemoradiation predicts long-term outcome. Ann Surg Oncol 2004; 11:955–61
Zirbes TK, Baldus SE, Moenig SP, et al. Prognostic impact of p21/waf1/cip1 in colorectal cancer. Int J Cancer 2000; 89:14–8
Buglioni S, D’Agnano I, Cosimelli M, et al. Evaluation of multiple bio-pathological factors in colorectal adenocarcinomas: independent prognostic role of p53 and bcl-2. Int J Cancer 1999; 84:545–52
Maeda K, Chung Y, Kang S, et al. Cyclin D1 overexpression and prognosis in colorectal adenocarcinoma. Oncology 1998; 55:145–51
Petrowsky H, Sturm I, Graubitz O, et al. Relevance of Ki-67 antigen expression and K-ras mutation in colorectal liver metastases. Eur J Surg Oncol 2001; 27:80–7
Jung C, Motwani M, Kortmansky J, et al. The cyclin-dependent kinase inhibitor flavopiridol potentiates gamma-irradiation-induced apoptosis in colon and gastric cancer cells. Clin Cancer Res 2003; 9(16 Pt 1):6052–61
Schwartz GK, O’Reilly E, Ilson D, et al. Phase I study of the cyclin-dependent kinase inhibitor flavopiridol in combination with paclitaxel in patients with advanced solid tumors. J Clin Oncol 2002; 20:2157–70
Acknowledgments
Supported by National Institutes of Health grant RO1 82534-01 (J.G.G.).
Author information
Authors and Affiliations
Corresponding author
Additional information
F.S. and D.B.C. contributed equally to this study.
Presented in part at the 58th Annual Cancer Symposium, Society of Surgical Oncology, Atlanta, Georgia, March 3–6, 2005.
Rights and permissions
About this article
Cite this article
Stipa, F., Chessin, D.B., Shia, J. et al. A Pathologic Complete Response of Rectal Cancer to Preoperative Combined-Modality Therapy Results in Improved Oncological Outcome Compared With Those Who Achieve No Downstaging on the Basis of Preoperative Endorectal Ultrasonography. Ann Surg Oncol 13, 1047–1053 (2006). https://doi.org/10.1245/ASO.2006.03.053
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/ASO.2006.03.053