-
PDF
- Split View
-
Views
-
Cite
Cite
Winfried V. Kern, Petra Steinke, Anja Schumacher, Sabine Schuster, Heike von Baum, Jürgen A. Bohnert, Effect of 1-(1-naphthylmethyl)-piperazine, a novel putative efflux pump inhibitor, on antimicrobial drug susceptibility in clinical isolates of Escherichia coli, Journal of Antimicrobial Chemotherapy, Volume 57, Issue 2, February 2006, Pages 339–343, https://doi.org/10.1093/jac/dki445
Close - Share Icon Share
Abstract
Objectives: 1-(1-Naphthylmethyl)-piperazine (NMP) has been shown to reverse multidrug resistance (MDR) in Escherichia coli overexpressing resistance–nodulation–cell division type efflux pumps, but there is no data on its activity in clinical isolates of E. coli.
Methods: The antimicrobial susceptibility of 60 clinical isolates of E. coli to a variety of antimicrobial agents was determined in the absence and presence of NMP and, for comparison, of Phe-Arg-β-naphthylamide (PAβN), another putative efflux pump inhibitor (EPI). The intracellular accumulation of ethidium bromide was measured to confirm efflux pump inhibition as the likely mechanism of action of NMP.
Results: Based on a 4-fold or greater reduction of the MIC after the addition of NMP in >50% of the isolates, significant effects of NMP at a concentration of 100 mg/L were seen for levofloxacin, linezolid and ethidium bromide. The ethidium bromide MIC changes after NMP addition correlated with differences in the ethidium bromide intracellular accumulation as measured by fluorometry in whole cell accumulation experiments. The activity of PAβN was different from that of NMP, in particular regarding macrolide resistance reversal, suggesting different modes of action of the two putative EPIs.
Conclusions: NMP is moderately active in reversing MDR in clinical isolates of E. coli and can partially restore fluoroquinolone susceptibility through inhibition of efflux pumps.
Introduction
Multidrug resistance (MDR) in Gram-negative bacteria may be caused by overexpression of resistance–nodulation–cell division (RND) type efflux pumps that are able to extrude various chemically unrelated compounds.1 MDR phenotypes can be selected easily in a variety of bacterial species by exposure to fluoroquinolones. For example, exposure of Escherichia coli to fluoroquinolones leads to first step mutations in the gyrase subunit A target gene, whereas repeated exposure to fluoroquinolones generates an MDR phenotype conferred primarily by enhanced activity of MDR efflux pumps. Similar observations were made in other Enterobacteriaceae and in several non-fermenters.2 Pharmacological MDR efflux pump inhibition has thus become an attractive research area. The high-throughput screening of a small molecule library in Pseudomonas aeruginosa led to the discovery of diverse dipeptide naphthylamides [one of the first prototypes being Phe-Arg-β-naphthylamide (PAβN)] acting as broad-spectrum efflux pump inhibitors (EPIs) in a variety of Gram-negative bacteria.3 Several arylpiperidines and other compounds capable of reversing MDR in E. coli and other Enterobacteriaceae have also been described.4 We have identified 1-(1-naphthylmethyl)-piperazine (NMP) as moderately active to reverse MDR in E. coli overexpressing RND type efflux pumps, but not in pump-deficient mutants, and to increase the intracellular concentration of ethidium bromide and levofloxacin, suggesting efflux pump inhibition as the mechanism of action.5 NMP reduced by 4-fold or more the MICs of levofloxacin, several other fluoroquinolones, tetracycline, chloramphenicol, linezolid, macrolides and oxacillin. We thought it useful early on in the development of an EPI to evaluate the potential of a lead/class of compounds to increase the susceptibility of clinical isolates to antibiotics effluxed by the pump. Consequently, the present work was done to test the MDR reversal activity of NMP in clinical isolates of E. coli and to compare it with the effects of PAβN.
Materials and methods
Bacterial strains
Laboratory strains included RND type pump-deficient strains and acrAB or acrEF overexpressing strains (Table 1). E. coli 2-DC14PS was an acrEF overexpressing mutant selected by ofloxacin.6 To confirm that acrEF overexpression was in fact the only relevant difference between 1-DC14PS and 2-DC14PS we inactivated acrEF by chromosomal deletion using the phage λ-based Red/ET homologous recombination system (Gene Bridges, Dresden, Germany),7 and obtained E12 representing an acrAB/acrEF double knockout strain (Table 1). Based on antimicrobial susceptibility tests there were no differences between 1-DC14PS and E12 (data not shown). Strains HS414 and HS276 (pump deficient) were gifts from M. Sulavik.8
Laboratory strains and clinical isolates of E. coli used in this study and corresponding intrinsic inhibitory effects of the putative EPIs NMP and PAβN, and of selected dyes
| . | . | . | MIC (mg/L) . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Strain . | Characteristics or number (n) . | Source/reference . | NMP . | PAβN . | ethidium bromidea . | pyronin Ya . | |||
| 3-AG100MKX | acrAB overexpressing gyrA mutant derived from AG100MKX (AG100 marA::Kmr) | 6 | 400 | 800 | 256b | 32c | |||
| 1-DC14PS | gyrA mutant derived from DC14 (AG100 ΔacrAB::Kmr) after selection with ofloxacin | 6 | 400 | 100 | 8 | 4 | |||
| 2-DC14PS | acrEF overexpressing mutant derived from 1-DC14PS after selection with ofloxacin | 6 | 400 | 800 | 512b | 32c | |||
| E12 | 2-DC14PS ΔacrEF | this study | 400 | 100 | 8 | 4 | |||
| HS414 | wild-type W3110 | 8 | 400 | 400 | 512b | 32c | |||
| HS276 | HS414 ΔacrEF ΔacrAB ΔyhiUV ΔacrD ΔyegMNO::Kmr | 8 | 400 | 50 | 8 | 1 | |||
| Clinical isolates | n = 60 | 800d (400 to >800) | 800d (100 to >800) | 256d | 32d | ||||
| . | . | . | MIC (mg/L) . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Strain . | Characteristics or number (n) . | Source/reference . | NMP . | PAβN . | ethidium bromidea . | pyronin Ya . | |||
| 3-AG100MKX | acrAB overexpressing gyrA mutant derived from AG100MKX (AG100 marA::Kmr) | 6 | 400 | 800 | 256b | 32c | |||
| 1-DC14PS | gyrA mutant derived from DC14 (AG100 ΔacrAB::Kmr) after selection with ofloxacin | 6 | 400 | 100 | 8 | 4 | |||
| 2-DC14PS | acrEF overexpressing mutant derived from 1-DC14PS after selection with ofloxacin | 6 | 400 | 800 | 512b | 32c | |||
| E12 | 2-DC14PS ΔacrEF | this study | 400 | 100 | 8 | 4 | |||
| HS414 | wild-type W3110 | 8 | 400 | 400 | 512b | 32c | |||
| HS276 | HS414 ΔacrEF ΔacrAB ΔyhiUV ΔacrD ΔyegMNO::Kmr | 8 | 400 | 50 | 8 | 1 | |||
| Clinical isolates | n = 60 | 800d (400 to >800) | 800d (100 to >800) | 256d | 32d | ||||
In none of the laboratory strains was there a ≥4-fold MIC reduction by the addition of NMP or PAβN at a concentration of 25 mg/L.
MIC reduction (≥4-fold) by the addition of NMP at a concentration of 100 mg/L.
MIC reduction (≥4-fold) by the addition of PAβN at a concentration of 100 mg/L.
Median value (and range).
Laboratory strains and clinical isolates of E. coli used in this study and corresponding intrinsic inhibitory effects of the putative EPIs NMP and PAβN, and of selected dyes
| . | . | . | MIC (mg/L) . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Strain . | Characteristics or number (n) . | Source/reference . | NMP . | PAβN . | ethidium bromidea . | pyronin Ya . | |||
| 3-AG100MKX | acrAB overexpressing gyrA mutant derived from AG100MKX (AG100 marA::Kmr) | 6 | 400 | 800 | 256b | 32c | |||
| 1-DC14PS | gyrA mutant derived from DC14 (AG100 ΔacrAB::Kmr) after selection with ofloxacin | 6 | 400 | 100 | 8 | 4 | |||
| 2-DC14PS | acrEF overexpressing mutant derived from 1-DC14PS after selection with ofloxacin | 6 | 400 | 800 | 512b | 32c | |||
| E12 | 2-DC14PS ΔacrEF | this study | 400 | 100 | 8 | 4 | |||
| HS414 | wild-type W3110 | 8 | 400 | 400 | 512b | 32c | |||
| HS276 | HS414 ΔacrEF ΔacrAB ΔyhiUV ΔacrD ΔyegMNO::Kmr | 8 | 400 | 50 | 8 | 1 | |||
| Clinical isolates | n = 60 | 800d (400 to >800) | 800d (100 to >800) | 256d | 32d | ||||
| . | . | . | MIC (mg/L) . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Strain . | Characteristics or number (n) . | Source/reference . | NMP . | PAβN . | ethidium bromidea . | pyronin Ya . | |||
| 3-AG100MKX | acrAB overexpressing gyrA mutant derived from AG100MKX (AG100 marA::Kmr) | 6 | 400 | 800 | 256b | 32c | |||
| 1-DC14PS | gyrA mutant derived from DC14 (AG100 ΔacrAB::Kmr) after selection with ofloxacin | 6 | 400 | 100 | 8 | 4 | |||
| 2-DC14PS | acrEF overexpressing mutant derived from 1-DC14PS after selection with ofloxacin | 6 | 400 | 800 | 512b | 32c | |||
| E12 | 2-DC14PS ΔacrEF | this study | 400 | 100 | 8 | 4 | |||
| HS414 | wild-type W3110 | 8 | 400 | 400 | 512b | 32c | |||
| HS276 | HS414 ΔacrEF ΔacrAB ΔyhiUV ΔacrD ΔyegMNO::Kmr | 8 | 400 | 50 | 8 | 1 | |||
| Clinical isolates | n = 60 | 800d (400 to >800) | 800d (100 to >800) | 256d | 32d | ||||
In none of the laboratory strains was there a ≥4-fold MIC reduction by the addition of NMP or PAβN at a concentration of 25 mg/L.
MIC reduction (≥4-fold) by the addition of NMP at a concentration of 100 mg/L.
MIC reduction (≥4-fold) by the addition of PAβN at a concentration of 100 mg/L.
Median value (and range).
Clinical non-duplicate isolates of E. coli were obtained from the clinical microbiology laboratories of Freiburg and Ulm University Hospitals. The isolates were not representative for consecutively isolated non-duplicate strains, but were ‘enriched’ to have a sufficient number of fluoroquinolone-resistant (defined as an MIC of levofloxacin >4 mg/L) and MDR clinical isolates.
Chemicals and media
PAβN, pyronin Y and CCCP (carbonylcyanide-3-chlorophenyl hydrazone) were purchased from Sigma–Aldrich (Steinheim, Germany), and NMP was obtained from Chess (Mannheim, Germany). Luria–Bertani (LB) broth and agar were obtained from Oxoid (Basingstoke, UK). Ethidium bromide was from Merck (Darmstadt, Germany).
Susceptibility testing
Susceptibilities to a panel of different antibiotics were studied by microbroth dilution in the presence or absence of NMP or PAβN, in accordance with NCCLS performance and interpretive guidelines. Custom microtitre plates containing selected antimicrobials at increasing concentrations were purchased from Merlin Diagnostics (Bornheim, Germany). The drugs tested included levofloxacin, tetracycline, chloramphenicol, oxacillin, linezolid, clarithromycin and rifampicin. A 4-fold or greater reduction in the MIC values after the addition of NMP or PAβN was considered significant. Microdilution tests were also performed to determine the MIC of ethidium bromide and pyronin Y. Pyronin Y was included as test dye after previous observations in P. aeruginosa that showed ethidium bromide to be unsuitable for the evaluation of PAβN as an EPI in this species.3 We used streptomycin as a control drug in the experiments, since this agent is not a substrate of commonly expressed MDR efflux pumps.9
Ethidium bromide whole cell accumulation assays
Cells were grown overnight on LB agar plates and diluted in 1 mL of PBS + 0.4% glucose (pH 7.4) until an optical density at 600 nm of ∼1 was reached. The cells were then transferred to a 96-well plate and NMP was added. Thereafter, ethidium bromide was added to a final concentration of 1 mg/L, and the relative fluorescence intensity was measured over time in a Safire (Tecan, Crailsheim, Germany) fluorescence plate-reader (excitation 518 nm, emission 605 nm). This assay showed large differences in ethidium bromide accumulation between RND pump-deficient and wild-type or acrAB and acrEF overexpressing strains (data not shown) that correlated with the large differences in the ethidium bromide MIC between the test strains (Table 1).
Results and discussion
Effects of NMP in laboratory strains
The concentration of NMP sufficient to reduce the MIC of levofloxacin by at least 4-fold in acrAB or acrEF overexpressing E. coli was 50 mg/L, and 100 mg/L was required to reduce by at least 4-fold the MIC of oxacillin, rifampicin, chloramphenicol and clarithromycin.5 At this concentration, NMP had no intrinsic inhibitory activity against pump-deficient strains 1-DC14PS, E12 and HS276 (Table 1), and its MIC was similar in the corresponding pump-overexpressing or wild-type strains. In contrast, the MIC of PAβN against the pump-deficient strains was much lower (50–100 mg/L) than that against the corresponding pump-overexpressing or wild-type strains (400–800 mg/L, Table 1). Differences in potency between the two putative EPIs, thus, may in part be related to differential efflux of the inhibitors themselves, and may be confounded by more toxic effects in cells that have low efflux competence.
Earlier experiments with laboratory strains 3-AG100MKX and 2-DC14PS showed a significant effect of NMP on the ethidium bromide MIC.5 In the present study, this observation was confirmed using the strain HS414. PAβN, in contrast, was unable to reduce the MIC of ethidium bromide, but a moderate effect by PAβN on the MIC of pyronin Y could be demonstrated (Table 1). This differential ability of the two EPIs to reduce the MICs of the two dyes, ethidium bromide and pyronin Y, is remarkable and has not previously been reported.
Changes in antimicrobial susceptibility of clinical isolates
NMP had no intrinsic antibacterial activity against clinical isolates at the concentration used in the MIC reduction experiments (Table 1). NMP at a concentration of 25 mg/L had no or minor effects on the MICs of the test drugs, whereas PAβN at this concentration reduced the MICs of linezolid, clarithromycin and rifampicin by 4-fold or more in the majority of clinical isolates irrespective of whether they were fluoroquinolone-resistant or fluoroquinolone-susceptible (Table 2). At this concentration PAβN had rather limited effects on fluoroquinolone MICs in E. coli, a finding consistent with a report by Saenz et al.10 Using ciprofloxacin instead of levofloxacin did not improve the capacity of PAβN to reverse fluoroquinolone resistance (data not shown). Particularly strong effects with PAβN at 25 mg/L were seen with clarithromycin and, surprisingly, with rifampicin. Rifampicin is a rather poor substrate of E. coli RND type efflux pumps given ∼2- to 4-fold differences in MIC between pump-deficient and pump-overexpressing strains,8,9 and the strong effect of PAβN on its MIC is not limited to pump-overexpressing strains.5 This suggests an effect of PAβN independent of (known) RND pumps or on membrane permeability.
Effects of the putative EPIs NMP and PAβN at two different concentrations on the MICs of different antimicrobial agents and dyes in clinical isolates of E. coli
| . | . | . | . | No. of isolates (%) with indicated fold reduction of MIC after the addition of . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | NMP . | . | . | . | PAβN . | . | . | . | |||||||
| . | . | . | . | 25 mg/L . | . | 100 mg/L . | . | 25 mg/L . | . | 100 mg/L . | . | |||||||
| Species/FQ susceptibility . | n . | Drug . | MIC50 (mg/L) . | ≥4-fold . | ≥16-fold . | ≥4-fold . | ≥16-fold . | ≥4-fold . | ≥16-fold . | ≥4-fold . | ≥16-fold . | |||||||
| E. coli, FQ-susceptible | 23 | levofloxacin | 0.0625 | 1 | – | 12 (52%) | 1 | 8 (35%) | – | 14 (61%) | 2 | |||||||
| tetracycline | 2 | – | – | 6 (26%) | 1 | 3 (13%) | – | 3 (13%) | – | |||||||||
| chloramphenicol | 4 | – | – | 2 | – | 3 (13%) | – | 5 (21%) | 1 | |||||||||
| linezolid | 128 | 2 | – | 17 (74%) | 8 (35%) | 14 (61%) | 2 | 15 (65%) | 2 | |||||||||
| oxacillin | 128 | 1 | – | 9 (39%) | – | 8 (35%) | 1 | 12 (52%) | 1 | |||||||||
| clarithromycin | 64 | – | – | 2 | – | 22 (96%) | 20 (87%) | 23 (100%) | 23 (100%) | |||||||||
| rifampicin | 8 | – | – | 9 (39%) | – | 22 (96%) | 22 (96%) | 23 (100%) | 23 (100%) | |||||||||
| ethidium bromide | 256 | – | – | 19 (83%) | – | – | – | 2 | – | |||||||||
| pyronin Y | 32 | 2 | – | – | – | 5 (22%) | – | 22 (96%) | 1 | |||||||||
| streptomycin | 16 | – | – | – | – | 2 | – | 2 | 2 | |||||||||
| E. coli, FQ-resistant | 37 | levofloxacin | 16 | 1 | – | 28 (76%) | – | 18 (49%) | – | 32 (87%) | 1 | |||||||
| tetracycline | 128 | 1 | – | 15 (41%) | 1 | 24 (65%) | 1 | 30 (81%) | 1 | |||||||||
| chloramphenicol | 32 | 1 | – | 15 (41%) | – | 14 (38%) | – | 22 (59%) | – | |||||||||
| linezolid | 256 | 5 (14%) | – | 28 (76%) | 24 (65%) | 30 (81%) | 12 (24%) | 29 (78%) | 24 (65%) | |||||||||
| oxacillin | 512 | – | – | 13 (35%) | 1 | 17 (46%) | – | 19 (51%) | 1 | |||||||||
| clarithromycin | 128 | – | – | 2 | – | 36 (97%) | 34 (92%) | 37 (100%) | 37 (100%) | |||||||||
| rifampicin | 16 | – | – | 22 (59%) | – | 36 (97%) | 36 (97%) | 37 (100%) | 37 (100%) | |||||||||
| ethidium bromide | 256 | 2 | – | 32 (86%) | – | – | – | 9 (24%) | – | |||||||||
| pyronin Y | 32 | – | – | – | – | 3 | – | 24 (65%) | – | |||||||||
| streptomycin | 1024 | – | – | 1 | – | – | – | – | – | |||||||||
| . | . | . | . | No. of isolates (%) with indicated fold reduction of MIC after the addition of . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | NMP . | . | . | . | PAβN . | . | . | . | |||||||
| . | . | . | . | 25 mg/L . | . | 100 mg/L . | . | 25 mg/L . | . | 100 mg/L . | . | |||||||
| Species/FQ susceptibility . | n . | Drug . | MIC50 (mg/L) . | ≥4-fold . | ≥16-fold . | ≥4-fold . | ≥16-fold . | ≥4-fold . | ≥16-fold . | ≥4-fold . | ≥16-fold . | |||||||
| E. coli, FQ-susceptible | 23 | levofloxacin | 0.0625 | 1 | – | 12 (52%) | 1 | 8 (35%) | – | 14 (61%) | 2 | |||||||
| tetracycline | 2 | – | – | 6 (26%) | 1 | 3 (13%) | – | 3 (13%) | – | |||||||||
| chloramphenicol | 4 | – | – | 2 | – | 3 (13%) | – | 5 (21%) | 1 | |||||||||
| linezolid | 128 | 2 | – | 17 (74%) | 8 (35%) | 14 (61%) | 2 | 15 (65%) | 2 | |||||||||
| oxacillin | 128 | 1 | – | 9 (39%) | – | 8 (35%) | 1 | 12 (52%) | 1 | |||||||||
| clarithromycin | 64 | – | – | 2 | – | 22 (96%) | 20 (87%) | 23 (100%) | 23 (100%) | |||||||||
| rifampicin | 8 | – | – | 9 (39%) | – | 22 (96%) | 22 (96%) | 23 (100%) | 23 (100%) | |||||||||
| ethidium bromide | 256 | – | – | 19 (83%) | – | – | – | 2 | – | |||||||||
| pyronin Y | 32 | 2 | – | – | – | 5 (22%) | – | 22 (96%) | 1 | |||||||||
| streptomycin | 16 | – | – | – | – | 2 | – | 2 | 2 | |||||||||
| E. coli, FQ-resistant | 37 | levofloxacin | 16 | 1 | – | 28 (76%) | – | 18 (49%) | – | 32 (87%) | 1 | |||||||
| tetracycline | 128 | 1 | – | 15 (41%) | 1 | 24 (65%) | 1 | 30 (81%) | 1 | |||||||||
| chloramphenicol | 32 | 1 | – | 15 (41%) | – | 14 (38%) | – | 22 (59%) | – | |||||||||
| linezolid | 256 | 5 (14%) | – | 28 (76%) | 24 (65%) | 30 (81%) | 12 (24%) | 29 (78%) | 24 (65%) | |||||||||
| oxacillin | 512 | – | – | 13 (35%) | 1 | 17 (46%) | – | 19 (51%) | 1 | |||||||||
| clarithromycin | 128 | – | – | 2 | – | 36 (97%) | 34 (92%) | 37 (100%) | 37 (100%) | |||||||||
| rifampicin | 16 | – | – | 22 (59%) | – | 36 (97%) | 36 (97%) | 37 (100%) | 37 (100%) | |||||||||
| ethidium bromide | 256 | 2 | – | 32 (86%) | – | – | – | 9 (24%) | – | |||||||||
| pyronin Y | 32 | – | – | – | – | 3 | – | 24 (65%) | – | |||||||||
| streptomycin | 1024 | – | – | 1 | – | – | – | – | – | |||||||||
FQ, fluoroquinolone.
Significant effects in >50% of isolates are indicated in boldface.
Effects of the putative EPIs NMP and PAβN at two different concentrations on the MICs of different antimicrobial agents and dyes in clinical isolates of E. coli
| . | . | . | . | No. of isolates (%) with indicated fold reduction of MIC after the addition of . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | NMP . | . | . | . | PAβN . | . | . | . | |||||||
| . | . | . | . | 25 mg/L . | . | 100 mg/L . | . | 25 mg/L . | . | 100 mg/L . | . | |||||||
| Species/FQ susceptibility . | n . | Drug . | MIC50 (mg/L) . | ≥4-fold . | ≥16-fold . | ≥4-fold . | ≥16-fold . | ≥4-fold . | ≥16-fold . | ≥4-fold . | ≥16-fold . | |||||||
| E. coli, FQ-susceptible | 23 | levofloxacin | 0.0625 | 1 | – | 12 (52%) | 1 | 8 (35%) | – | 14 (61%) | 2 | |||||||
| tetracycline | 2 | – | – | 6 (26%) | 1 | 3 (13%) | – | 3 (13%) | – | |||||||||
| chloramphenicol | 4 | – | – | 2 | – | 3 (13%) | – | 5 (21%) | 1 | |||||||||
| linezolid | 128 | 2 | – | 17 (74%) | 8 (35%) | 14 (61%) | 2 | 15 (65%) | 2 | |||||||||
| oxacillin | 128 | 1 | – | 9 (39%) | – | 8 (35%) | 1 | 12 (52%) | 1 | |||||||||
| clarithromycin | 64 | – | – | 2 | – | 22 (96%) | 20 (87%) | 23 (100%) | 23 (100%) | |||||||||
| rifampicin | 8 | – | – | 9 (39%) | – | 22 (96%) | 22 (96%) | 23 (100%) | 23 (100%) | |||||||||
| ethidium bromide | 256 | – | – | 19 (83%) | – | – | – | 2 | – | |||||||||
| pyronin Y | 32 | 2 | – | – | – | 5 (22%) | – | 22 (96%) | 1 | |||||||||
| streptomycin | 16 | – | – | – | – | 2 | – | 2 | 2 | |||||||||
| E. coli, FQ-resistant | 37 | levofloxacin | 16 | 1 | – | 28 (76%) | – | 18 (49%) | – | 32 (87%) | 1 | |||||||
| tetracycline | 128 | 1 | – | 15 (41%) | 1 | 24 (65%) | 1 | 30 (81%) | 1 | |||||||||
| chloramphenicol | 32 | 1 | – | 15 (41%) | – | 14 (38%) | – | 22 (59%) | – | |||||||||
| linezolid | 256 | 5 (14%) | – | 28 (76%) | 24 (65%) | 30 (81%) | 12 (24%) | 29 (78%) | 24 (65%) | |||||||||
| oxacillin | 512 | – | – | 13 (35%) | 1 | 17 (46%) | – | 19 (51%) | 1 | |||||||||
| clarithromycin | 128 | – | – | 2 | – | 36 (97%) | 34 (92%) | 37 (100%) | 37 (100%) | |||||||||
| rifampicin | 16 | – | – | 22 (59%) | – | 36 (97%) | 36 (97%) | 37 (100%) | 37 (100%) | |||||||||
| ethidium bromide | 256 | 2 | – | 32 (86%) | – | – | – | 9 (24%) | – | |||||||||
| pyronin Y | 32 | – | – | – | – | 3 | – | 24 (65%) | – | |||||||||
| streptomycin | 1024 | – | – | 1 | – | – | – | – | – | |||||||||
| . | . | . | . | No. of isolates (%) with indicated fold reduction of MIC after the addition of . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | NMP . | . | . | . | PAβN . | . | . | . | |||||||
| . | . | . | . | 25 mg/L . | . | 100 mg/L . | . | 25 mg/L . | . | 100 mg/L . | . | |||||||
| Species/FQ susceptibility . | n . | Drug . | MIC50 (mg/L) . | ≥4-fold . | ≥16-fold . | ≥4-fold . | ≥16-fold . | ≥4-fold . | ≥16-fold . | ≥4-fold . | ≥16-fold . | |||||||
| E. coli, FQ-susceptible | 23 | levofloxacin | 0.0625 | 1 | – | 12 (52%) | 1 | 8 (35%) | – | 14 (61%) | 2 | |||||||
| tetracycline | 2 | – | – | 6 (26%) | 1 | 3 (13%) | – | 3 (13%) | – | |||||||||
| chloramphenicol | 4 | – | – | 2 | – | 3 (13%) | – | 5 (21%) | 1 | |||||||||
| linezolid | 128 | 2 | – | 17 (74%) | 8 (35%) | 14 (61%) | 2 | 15 (65%) | 2 | |||||||||
| oxacillin | 128 | 1 | – | 9 (39%) | – | 8 (35%) | 1 | 12 (52%) | 1 | |||||||||
| clarithromycin | 64 | – | – | 2 | – | 22 (96%) | 20 (87%) | 23 (100%) | 23 (100%) | |||||||||
| rifampicin | 8 | – | – | 9 (39%) | – | 22 (96%) | 22 (96%) | 23 (100%) | 23 (100%) | |||||||||
| ethidium bromide | 256 | – | – | 19 (83%) | – | – | – | 2 | – | |||||||||
| pyronin Y | 32 | 2 | – | – | – | 5 (22%) | – | 22 (96%) | 1 | |||||||||
| streptomycin | 16 | – | – | – | – | 2 | – | 2 | 2 | |||||||||
| E. coli, FQ-resistant | 37 | levofloxacin | 16 | 1 | – | 28 (76%) | – | 18 (49%) | – | 32 (87%) | 1 | |||||||
| tetracycline | 128 | 1 | – | 15 (41%) | 1 | 24 (65%) | 1 | 30 (81%) | 1 | |||||||||
| chloramphenicol | 32 | 1 | – | 15 (41%) | – | 14 (38%) | – | 22 (59%) | – | |||||||||
| linezolid | 256 | 5 (14%) | – | 28 (76%) | 24 (65%) | 30 (81%) | 12 (24%) | 29 (78%) | 24 (65%) | |||||||||
| oxacillin | 512 | – | – | 13 (35%) | 1 | 17 (46%) | – | 19 (51%) | 1 | |||||||||
| clarithromycin | 128 | – | – | 2 | – | 36 (97%) | 34 (92%) | 37 (100%) | 37 (100%) | |||||||||
| rifampicin | 16 | – | – | 22 (59%) | – | 36 (97%) | 36 (97%) | 37 (100%) | 37 (100%) | |||||||||
| ethidium bromide | 256 | 2 | – | 32 (86%) | – | – | – | 9 (24%) | – | |||||||||
| pyronin Y | 32 | – | – | – | – | 3 | – | 24 (65%) | – | |||||||||
| streptomycin | 1024 | – | – | 1 | – | – | – | – | – | |||||||||
FQ, fluoroquinolone.
Significant effects in >50% of isolates are indicated in boldface.
Increasing the NMP concentration to 100 mg/L enhanced its activity and gave effects that were expected from the above results in the laboratory test strains. For levofloxacin, linezolid and ethidium bromide, a 4-fold or greater reduction in the MIC was now observed in the majority of isolates (Table 2). Enhanced effects compared with the lower concentration of 25 mg/L were also seen for other drugs but were more often seen in fluoroquinolone-resistant isolates. NMP had no significant effects on the MIC of pyronin Y. This was unexpected because pyronin Y intracellular accumulation (as measured by fluorometry, data not shown) and pyronin Y MICs were quite different for pump-deficient and corresponding overexpressing or wild-type E. coli laboratory strains (Table 1). The comparatively poor activity of NMP on the clarithromycin MIC in clinical isolates was also surprising given previous evidence showing that macrolides are substrates of RND type efflux pumps.5,8,911 In most fluoroquinolone-resistant isolates (51%) the activity of NMP was sufficient to render isolates drug-susceptible at clinically achievable concentrations. This was unlike the NMP effect on the MIC of linezolid which, despite the consistent resistance lowering activity of NMP, remained above the breakpoint for resistance (>4 mg/L).
Increasing the PAβN concentration to 100 mg/L enhanced its effect on the reduction of the levofloxacin MIC to a potency that was similar to NMP at 100 mg/L. At this concentration, PAβN also more frequently reduced the MIC of pyronin Y, but remained ineffective in reducing the MIC of ethidium bromide (Table 2), similar to observations made with laboratory strains. Ineffectiveness of PAβN to reverse resistance of ethidium bromide has also been observed in P. aeruginosa.3
NMP and intracellular ethidium bromide accumulation
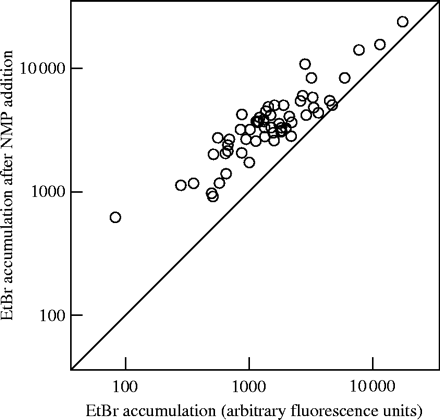
Ethidium bromide accumulation assays have previously been used to demonstrate effects of putative EPIs on intracellular substrate accumulation. Using the fluorometric assay as described, we have previously shown that NMP increased the intracellular ethidium bromide concentration in E. coli 3-AG100MKX in a dose-dependent manner.5 When applied to our collection of E. coli clinical isolates, mean fluorescence (± SD) without EPI at 25 min was 2152 ± 2730 arbitrary fluorescence units (AU). NMP addition (100 mg/L) increased fluorescence to 4366 ± 3752 AU (mean increase 2214 AU) (Figure 1). The difference between fluorescence with and without NMP addition was highly significant (P < 0.001, Kruskall–Wallis test). The ethidium bromide accumulation values correlated inversely with its MIC values (Pearson correlation coefficient −0.28; P = 0.02).
Ethidium bromide (EtBr) fluorescence at 25 min in a whole cell assay of 60 clinical isolates of E. coli with or without the addition of NMP (100 mg/L), a novel putative EPI. The mean increase in arbitrary fluorescence units was 2214. Results represent the mean of two independent experiments.
Conclusions
The above findings confirm that NMP has EPI activity in clinical isolates of E. coli and indicate that this activity is probably most relevant in fluoroquinolone-resistant isolates. Resistance reversal by NMP to clinically relevant MIC values was achieved for levofloxacin in a considerable proportion of resistant E. coli clinical isolates. Its activity to reverse the MIC of linezolid was notable but insufficient to render the isolates susceptible to this agent primarily designed for anti-Gram-positive coverage. Of interest were the differences found between NMP and PAβN in substrate preference which were most striking regarding ethidium bromide, pyronin Y, clarithromycin and rifampicin. This is consistent with the view that different antibiotics may have different binding sites on the pump12–14 with which the EPI (here NMP or PAβN) might interfere in a variable manner. Another explanation is that one of the two EPIs is active on other MDR efflux pumps accounting for the observed substrate-specific differences in potency. In a recent study, PAβN, for example, was actively inhibiting ketolide efflux independent of the AcrAB-TolC system.11 In another previous study, PAβN increased the permeability of the outer membrane in P. aeruginosa, but only in the absence of a functional MexAB-OprM efflux pump.3 It is of note that polycations like polymyxin B nonapeptide make E. coli and other Gram-negative species up to 100 times more susceptible specifically to rifampicin and macrolides.15 Perhaps such additional effects interfere with the relative pump inhibitory effect towards specific substrates. NMP and other available putative EPIs might be used to study in more detail the options to inhibit RND type efflux pumps and thereby enhance our understanding of the structure–activity relationships of EPIs.
Transparency declarations
None to declare.
This study was supported by the Landesstiftung Baden-Württemberg and by BMBF grant 01KI9951.
References
Lomovskaya O, Warren MS, Lee A et al. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy.
Pagès JM, Masi M, Barbe J. Inhibitors of efflux pumps in Gram-negative bacteria.
Bohnert JA, Kern WV. Selected arylpiperazines are capable of reversing multidrug resistance in Escherichia coli overexpressing RND efflux pumps.
Jellen-Ritter AS, Kern WV. Enhanced expression of the multidrug efflux pumps AcrAB and AcrEF associated with insertion element transposition in Escherichia coli mutants selected with a fluoroquinolone.
Zhang Y, Buchholz F, Muyrers JP et al. A new logic for DNA engineering using recombination in Escherichia coli.
Sulavik MC, Houseweart C, Cramer C et al. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes.
Nishino K, Yamaguchi A. Analysis of a complete library of putative drug transporter genes in Escherichia coli.
Saenz Y, Ruiz J, Zarazaga M et al. Effect of the efflux pump inhibitor Phe-Arg-β-naphthylamide on the MIC values of the quinolones, tetracycline and chloramphenicol, in Escherichia coli isolates of different origin.
Chollet R, Chevalier J, Bryskier A et al. The AcrAB-TolC pump is involved in macrolide resistance but not in telithromycin efflux in Enterobacter aerogenes and Escherichia coli.
Elkins CA, Nikaido H. Substrate specificity of the RND-type multidrug efflux pumps AcrB and AcrD of Escherichia coli is determined predominantly by two large periplasmic loops.
Mao W, Warren MS, Black DS et al. On the mechanism of substrate specificity by resistance nodulation division (RND)-type multidrug resistance pumps: the large periplasmic loops of MexD from Pseudomonas aeruginosa are involved in substrate recognition.
Yu EW, McDermott G, Zgurskaya HI et al. Structural basis of multiple drug-binding capacity of the AcrB multidrug efflux pump.
Author notes
1Center for Infectious Diseases and Travel Medicine, Department of Medicine, University Hospital, Freiburg, Germany; 2Section of Hospital Hygiene, Department of Medical Microbiology and Hygiene, University of Ulm, Ulm, Germany