Abstract
Despite significant advances in the use of surgery, chemotherapy and radiotherapy to treat squamous cell carcinoma of the head and neck (SCCHN), prognosis has improved little over the past 30 years. There is a clear need for novel, more effective therapies to prevent relapse, control metastases and improve overall survival. Improved understanding of SCCHN disease biology has led to the introduction of molecularly targeted treatment strategies in these cancers. The epidermal growth factor receptor (EGFR) is expressed at much higher levels in SCCHN tumours than in normal epithelial tissue, and EGFR expression correlates with poor prognosis. Therefore, much effort is currently directed toward targeting aberrant EGFR activity (e.g. cell signalling) in SCCHN. This review discusses the efficacy of novel therapies targeting the EGFR (e.g. anti-EGFR antibodies and EGFR tyrosine kinase inhibitors) that are currently tested in SCCHN patients.
Similar content being viewed by others
Main
Head and neck cancer (HNC) accounts for about 5% of all cancers with >500 000 cases diagnosed worldwide and >100 000 in Europe each year. The majority of HNC in the Western world is of squamous cell origin (>90% squamous cell carcinoma of the head and neck, SCCHN) and present with locally or regionally advanced disease (Rogers et al, 2005). Patients with early-stage disease are treated with surgery and/or radiotherapy and nearly 80% are cured. Chemotherapy added to locoregional treatment provides a demonstrated survival benefit in nonmetastatic SCCHN (Pignon et al, 2000). However, despite combined treatment approaches (surgery and radiation/chemoradiation therapy) most patients with resectable advanced disease develop local or regional recurrences (50–60%), metastatic disease (∼20%) or secondary primaries. Patients with unresectable advanced disease have a 5-year survival of <10% and recurrent/metastatic cases have a median survival of approximately 6–9 months, which has not changed significantly for 30 years. Several therapeutic options are available for these patients, including (re-)irradiation, salvage surgery, palliative chemotherapy or best supportive care for patients with low performance status. The most commonly used agents are cisplatin or carboplatin, often in combination with taxanes or 5-fluorouracil. Response rates (RR) to first-line platinum-based chemotherapy are only ∼30%. In recurrent/metastatic SCCHN, survival benefits of 10 weeks may be expected (Morton et al, 1985; Browman and Cronin, 1994). Although several combinations of classical chemotherapeutics have increased RR, improved survival has not been observed. Options and RR of patients refractory to platinum-based therapies are generally very poor. Therefore, there is clearly an unmet therapeutic need for new active, less toxic agents for SCCHN treatment.
Targeting epidermal growth factor- receptor signalling in SCCHN
Several molecularly targeted strategies have been evaluated in HNC patients owing to (1) mechanism of action, (2) greater selectivity and (3) different/lower toxicity. Potential targets are growth factor receptors, signal transduction, cell cycle control, protein degradation, hypoxia, angiogenesis and prostaglandin synthesis. The epidermal growth factor-receptor (EGFR) is a particularly interesting target as it plays an important role in regulation of cellular proliferation, differentiation and survival of epithelial cells and tumours of epithelial cell origin. Additionally, aberrant EGFR signalling imparts SCCHN cells with classic tumour cell characteristics, including decreased apoptosis, enhanced invasiveness, migration, angiogenesis and metastasis. Furthermore, EGFR is overexpressed in approximately 90–100% of SCCHN specimens and has been associated with worse prognosis, including advanced stage, poorly differentiated tumours and poor survival (Santini et al 1991; Dassonville et al, 1993). Epidermal growth factor-receptor is one of four transmembrane growth factor receptors that share structural and functional similarities, including EGFR (=HER1, c-erbB-1), HER-2/neu (c-erbB-2), HER3 (c-erbB-3) and HER4 (c-erbB-4). The EGFR, a 170 kDa glycoprotein, consisting of an extracellular domain, a transmembrane region and an intracellular domain with tyrosine kinase function, responds to numerous ligands, such as transforming growth factor alpha (TGF-α), betacellulin, amphiregulin, epiregulin, EGF and heparin-binding EGF (Rogers et al, 2005).
Downstream effects of EGFR activation after receptor dimerisation, internalisation and autophosphorylation are mediated through several signal transduction pathways involving the RAS/MAP kinase, the phosphatidylinositol 3-kinase (PI-3K)/Akt, the PLCγ and the JAK-STAT pathways (Rogers et al, 2005; Kalyankrishna and Grandis, 2006) (Figure 1). Although the main autophosphorylation sites in ErbB receptors recruit extensively overlapping molecules to the active receptors, preferential modulation of signalling pathways seems to occur (e.g. EGFRs with kinase-domain mutations preferentially activate the pro-survival PI-3K/AKT pathway and the STAT pathway). Downstream effectors of EGFR (e.g. ERK-1/2, AKT, STAT-3/5) are activated in SCCHN (Kalyankrishna and Grandis, 2006). Epidermal growth factor receptor ligand binding results in several homo- or heterodimeric complexes. Furthermore, EGFR can be activated by other receptor tyrosine kinases including insulin-like growth factor-1 receptor, adhesion molecules (e.g. E-cadherin and integrins) and G-protein-coupled receptors (GPCR).
Intracellular signalling of the EGFR. Shown are the major signalling pathways downstream of c-erbB-receptors (e.g. EGFR). Modified after Rogers et al (2005) and Kalyankrishna and Grandis (2006). Binding of specific ligands (e.g. EGF, heparin-binding EGF, TGF-α, amphiregulin, betacellulin and heregulin) may generate up to 10 types of homo- or heterodimeric complexes resulting in conformational changes in the intracellular EGFR kinase domain, which lead to autophosphorylation and activation. Consequently, signalling molecules, including growth factor receptor-bound protein-2 (Grb-2), Shc and IRS-1 are recruited to the plasma membrane. G-protein coupled receptors can also activate EGFR in a ligand-independent manner by Src-mediated direct phosphorylation of Y-845. Insulin-like growth factor-1 receptor can also transactivate the EGFR. Activation of several signalling cascades is triggered predominately by the RAS-to-MAPK and the PI-3K/Akt pathways, resulting in enhanced tumour growth, survival, invasion and metastasis.
Deregulation of EGFR function is a common feature in several human malignancies including lung, breast, colorectal, prostate and HNC. Mechanisms of EGFR activation include (1) receptor overexpression in most epithelial malignancies (EGFR in up to 90% of SCCHN; ErbB2 in 3–29%; ErbB3 in 21% and ErbB4 in 26%), (2) constitutively activated EGFR mutants, (3) autocrine activation by ligand overexpression (e.g. TGF-α), (4) ligand-independent activation through other receptor systems (e.g. ErbB2/HER2), (5) EGFR transactivation by GPCR-induced processing of transmembrane growth factor precursors by ADAM family metalloproteases, (6) gene amplification and/or (7) loss of negative regulatory mechanisms (Rogers et al, 2005; Kalyankrishna and Grandis, 2006).
Dysregulated p53, polymorphisms in dinucleotide repeats in intron 1 of the EGFR gene and EGFR amplification can all lead to increased EGFR mRNA synthesis. However, EGFR gene amplification was only observed in seven out of 33 patients with SCCHN and did not correlate with EGFR protein overexpression, suggesting that gene amplification is not pathogenetically involved in EFGR protein overexpression (Mrhalova et al, 2005). Furthermore, overexpression of cortactin may inhibit ligand-induced EGFR downregulation. Interestingly, tobacco smoke increases EGFR ligand levels (e.g. amphiregulin, TGF-α) culminating in EGFR activation and increased levels of cyclooxygenase 2 and prostaglandin E2, which can transactivate EGFR (Kalyankrishna and Grandis, 2006).
Recently, three identical in-frame deletions in exon 19 (E746_A750del) of the EGFR gene were reported in three out of 41 (7.3%) Korean SCCHN cases (Lee et al, 2005). In contrast, EGFR kinase domain mutations were rare among US (zero out of 65) or European (one out of 100) SCCHN cases (Cohen et al, 2005a; Loeffler-Ragg et al, 2006). Interestingly, one gefitinib-responsive SCCHN patient harboured a heterozygous mutation within ErbB2 (V773A) (Cohen et al, 2005a). ErbB2 heterodimerises with EGFR and ErbB2 mutations have recently been reported within a subset of non-small cell lung cancer (NSCLC). Epidermal growth factor receptor vIII, a deletion of exons 2–7 resulting in a truncated extracellular domain and constitutive tyrosine kinase activation, has been reported in glioblastoma multiforme (>50%; Moscatello et al, 1995), NSCLC (1–42%; Ji et al, 2006; Sonnweber et al, 2006) and in SCCHN (42%; Sok et al, 2006).
Inhibition of EGFR signalling
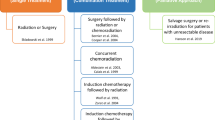
Several approaches to block EGFR signalling in human diseases have been tested, including (1) monoclonal antibodies (Mabs), (2) small molecule tyrosine kinase inhibitors (TKIs), (3) inhibition of receptor trafficking to the cell membrane and (4) inhibition of EGFR synthesis through antisense oligonucleotides. Only Mabs against EGFR and EGFR-specific TKIs have been evaluated in phase III trials (Figure 2). Inhibition of EGFR signalling has been used in primary treatment of locally advanced SCCHN with radiation therapy and as first/second-line agents in recurrent/metastatic SCCHN.
Preclinical and clinical development of Mabs and TKIs targeting the EGFR in SCCHN. The Mabs (light grey arrows), tested to date, include IMC-C225 (cetuximab), ICR62, ABX-EGF (panitumumab), EMD72000 (matuzumab), h-R3 (nimotuzumab), 2F8 (zalutumumab) and ch806. Cetuximab (IMC-C225) has been approved for use in SCCHN by both the FDA and EMEA in combination with radiotherapy. Nimotuzumab (h-R3) was recently approved for head and neck cancer in Argentina, Cuba, Columbia, China and India. ch806 is an EGFRvIII-specific Mab. Tyrosine kinase inhibitors (dark grey arrows) in clinical development include the EGFR inhibitors ZD1839 (gefitinib) and OSI-774 (erlotinib; formerly known as CP-358-774) as well as the EGFR/HER-2 inhibitor GW572016 (lapatinib).
Anti-EGFR Mabs
EGFR-specific Mabs and their characteristics are listed in Table 1. Mechanisms of action include (1) inhibition of receptor activation and signalling by blocking ligand binding to the extracellular domain, and (2) induction of antibody-dependent cell-mediated cytotoxicity and/or complement-dependent cytotoxicity.
Anti-EGFR Mabs in recurrent/metastatic SCCHN
Cetuximab (IMC-C225) has antitumour activity against several tumour cell lines expressing EGFR and in SCC tumour xenograft models. Cetuximab has been studied in several phase I–III trials in locoregionally advanced or recurrent/metastatic SCCHN patients (Table 2). In three phase I trials (including 26 SCCHN patients) of cetuximab as a single, weekly multiple dose (5–400 mg m−2) with or without cisplatin (60 mg m−2 once every 4 weeks), disease stabilisation was observed without reaching MTD. Nine out of 13 patients treated with cetuximab doses ⩾50 mg m−2 plus cisplatin completed 12 weeks of therapy including two partial responses (PRs) in SCCHN after treatment with 200 and 400 mg m−2 (Baselga et al, 2000). Another phase Ib study combining cetuximab with cisplatin in recurrent SCCHN reported two complete remissions (CRs) and four PRs of nine patients (Shin et al, 2001). The most frequently occurring adverse events (AEs) were fever/chills, asthenia, transaminase elevation, nausea and skin toxicities including acneiform rashes, flushing and seborrheic dermatitis. Grade 3–4 AEs included aseptic meningitis, allergic reaction, epiglottitis plus dyspnoe.
Antitumour activity of cetuximab plus cisplatin in platinum-refractory SCCHN patients was recently reported. In a multicentre phase II trial, 132 SCCHN patients were treated with two 3-week cycles of cisplatin/paclitaxel or cisplatin/5-fluorouracil (Herbst et al, 2005). Patients with a CR or PR continued standard therapy. Patients with stable disease (SD; n=51) or progressive disease (PD/1; n=25) received cetuximab plus cisplatin (75 or 100 mg m−2 every 3 weeks). Patients who developed PD within 90 days (PD/2; n=54) were subsequently enrolled to cetuximab plus cisplatin. Objective responses were observed in 5, 3 and 9 patients with a median response duration of 4.2, 4.1 and 7.4 months and a median overall survival (OS) of 6.1, 4.3 and 11.7 months for the PD/1, PD/2 and SD groups, respectively. The most common toxicities were anaemia, acne-like skin rash, leukopenia, fatigue/malaise and nausea/vomiting. Seven patients developed grade 3/4 hypersensitivity reactions to cetuximab (Herbst et al, 2005).
Baselga et al (2005) reported another multicentre phase II trial with 96 platinum-refractory SCCHN patients who received cetuximab plus cisplatin (⩾60 mg m−2 cycle−1) or carboplatin (⩾250 mg m−2 cycle−1). In the intent-to-treat population, the RR was 10% with a disease control rate (DCR=CR+PR+SD) of 53%. The median time to progression (TTP) was 85 days and OS 183 days, respectively. Treatment was well tolerated with skin reactions being the most common cetuximab-related event.
Cetuximab also exhibited single-agent activity in platinum-refractory SCCHN patients. In a multicentre phase II study with 103 evaluable patients, a 16.5% RR was reported. Median TTP and OS were 85 and 175 days, respectively (Trigo et al, 2004).
In a phase III randomised multicentre placebo-controlled ECOG trial in 117 metastatic/recurrent SCCHN patients, cetuximab plus cisplatin (100 mg m−2 every 4 weeks) was compared with cisplatin plus placebo (Burtness et al, 2005). The hazard ratio (HR) for progression (primary end point) for the combination compared to cisplatin plus placebo was 0.78 (95% CI, 0.54–1.12) with a median PFS of 4.2 vs 2.7 months (P=0.09), respectively. This was not significant as the study was powered to detect a 50% reduction in HRs. Furthermore, both arms had a significant drop-off rate and the control arm performed better than expected. Median OS was 9.2 months for cisplatin plus cetuximab and 8.0 months for cisplatin plus placebo (P=0.21) and the objective RR was 26 vs 10%, respectively (P=0.03). However, there was a survival advantage for the development of rash (HR for survival by skin toxicity in cetuximab-treated patients 0.42 (95% CI, 0.21–0.86)).
Zalutumumab (HuMax-EGFrR, 2F8) was recently tested in a phase I/II trial in 24 patients with recurrent/metastatic SCCHN (0.15–8 mg kg−1 iv, after 28 days 4 × weekly). Two PRs and eight SDs were observed in 15 evaluable patients all occurring in the 1, 2, 4 and 8 mg kg−1 dose group (Bastholt et al, 2005). In January 2006, FDA awarded Fast Track status to zalutumumab for HNC patients who previously failed standard therapies. A pivotal phase III study with zalutumumab in 273 SCCHN patients who are refractory to or intolerant of standard platinum-based chemotherapy was initiated in September 2006.
Anti-EGFR-Mabs in combination with radiotherapy
In a phase I study in locoregionally advanced SCCHN patients, cetuximab was delivered in combination with conventional or hyperfractionated RT (Robert et al, 2001). All patients achieved an objective response (13 CRs and two PRs). The recommended dose for phase II/III trials was 400–500 mg m−2 loading dose and 250 mg m−2 weekly maintenance dose. A pilot phase II study of concurrent cetuximab, cisplatin and concomitant boost radiotherapy for locoregionally advanced SCCHN patients (n=32) reported a 3-year OS of 75%, PFS of 56% and a locoregional control rate of 71%. However, the study was closed for significant AEs including two deaths (Pfister et al, 2006). A randomised phase III study compared radiation with or without cetuximab for patients with locally advanced inoperable SCCHN patients (Bonner et al, 2006). The median duration of locoregional control was 24.4 months among patients treated with cetuximab plus radiotherapy and 14.9 months among those given radiotherapy alone (HR for locoregional progression or death, 0.68; P=0.005). With a median follow-up of 54.0 months, the median OS was 49.0 months among patients treated with combined therapy and 29.3 months among those treated with radiotherapy alone (HR for death, 0.74; P=0.03). Radiotherapy plus cetuximab significantly prolonged PFS (HR for disease progression or death, 0.70; P=0.006). Cetuximab did not significantly add to the acute side effects of radiotherapy, offering a real therapeutic advantage to patients who are ineligible to receive standard chemoradiation. As a result of this study, cetuximab was approved by the FDA/EMEA in combination with radiotherapy to treat SCCHN in February and April 2006. However, this study did not compare cetuximab plus radiotherapy with platinum-based radiotherapy, which is the current standard of care. Additionally, radiotherapy was not uniformly administered among all patients. These shortcomings are currently addressed in RTOG trial 0522, which has been recently initiated and compares chemoradiation with cisplatin to chemoradiation plus cetuximab.
Nimotuzumab (h-R3) has demonstrated clinical benefit without rash development in several clinical trials. In a single-centre phase I/II trial with 24 locally advanced SCCHN patients who received 6 × weekly infusions (cumulative doses of 300, 600, 1200 and 2400 mg) plus radiotherapy (60–66 Gy), the combination was well-tolerated with no skin or allergic toxicity (Crombet et al, 2004). Nimotuzumab was recently approved for nasopharyngeal cancer in China (April 2005), based on a 75% improvement in CR (91 vs 52%) in a phase II trial in 130 patients diagnosed with squamous cell nasopharyngeal carcinoma who were treated with nimotuzumab plus radiotherapy vs radiotherapy alone. It has also been approved for the treatment of HNC in Argentina, Columbia, Cuba and India (July 2006). A phase III trial in HNC is currently ongoing.
EGFR TKIs
Numerous protein kinase inhibitors have been developed including inhibitors of the EGFR kinase domain. Some molecules are highly specific for EGFR (e.g. ZD1839, OSI-774), while others may block additional Erb family kinases (e.g. GW572016, PKI-66) or other protein kinase families (ZD6474). Both ZD1839 (Gefitinib) and OSI-774 (formerly known as CP-358-774, Erlotinib) have FDA approval for treatment of locally advanced or metastatic NSCLC since May 2003 and November 2004, respectively. Three orally active EGFR inhibitors have been tested in clinical trials in recurrent/metastatic SCCHN or in combination with radiotherapy in locoregionally advanced SCCHN (Table 3).
TKIs in recurrent/metastatic SCCHN
Gefitinib (IressaR, AstraZeneca Pharmaceuticals, London, UK) impeded in vitro and in vivo growth of cell lines that express high, intermediate or low levels of EGFR and high levels of HER-2. Furthermore, gefitinib has additive or synergistic properties in combination with cisplatin, carboplatin, paclitaxel, taxanes, doxorubicin and radiotherapy. A phase II trial of 500 mg gefitinib in 52 patients with recurrent/metastatic SCCHN reported an RR of 10.6% and a DCR of 53% (Table 3) (Cohen et al, 2003). Half the cohort received gefitinib as second-line therapy. Median TTP and OS were 3.4 and 8.1 months, respectively. The only grade 3 toxicity encountered was diarrhoea (n=3). Performance status and development of skin toxicity were found to be strong predictors of response, progression and survival. In another phase II trial, gefitinib (500 mg dose−1, reduction to 250 mg) was tested in 32 patients with recurrent SCCHN. In cohort A (no chemotherapy), three PR and six SD were observed out of 20 patients (clinical benefit in 45%). In cohort B (one previous chemotherapy), three out of 12 patients achieved SD (25% clinical benefit). There was no association between rash incidence/grade and clinical benefit (Wheeler et al, 2005). An expanded access program with gefitinib (500 mg day−1) in 47 SCCHN patients reported an 8% clinical RR with 36% DCR. The median TTP and OS were 2.6 and 4.3 months, respectively. Acneiform folliculitis was the most frequent toxicity observed (76%) (Kirby et al, 2006). In another phase II trial with gefitinib (250 mg day−1) in 70 SCCHN patients, two PRs and a 34% DCR were observed. Median TTP and OS were 1.8 and 5.5 months, respectively, with no difference between untreated and pretreated patients. Gefitinib monotherapy at 250 mg day−1 in recurrent/metastatic SCCHN may have less activity than was previously observed for 500 mg daily. Squamous cell carcinomas of the head and neck responses to gefitinib or erlotinib seem not to be linked to EGFR kinase mutational status, as these mutations are rare in this disease (Cohen et al, 2005a, 2005b). Recently, a phase I study in SCCHN reported that gefitinib (250/500 mg q.d.) in combination with celecoxib (200/400 mg b.i.d.) is very well tolerated in patients with incurable SCCHN (Wirth et al, 2005).
Erlotinib (TarcevaR; Roche, Genentech, OSI Pharmaceuticals) has FDA/EMEA approval as single-agent treatment for patients with locally advanced or metastatic NSCLC, and FDA approval in combination with gemcitabine for first-line treatment of patients with locally advanced, unresectable or metastatic pancreatic cancer. A multicentre phase II trial of erlotinib in 115 patients with recurrent and metastatic SCCHN reported a 4.3% overall RR, including five PRs (Soulieres et al, 2004). Disease stabilisation was maintained in 44 patients (38.3%) for a median duration of 16.1 weeks. Median PFS was 9.6 weeks and median OS was 6.0 months. Rash (79%) and diarrhoea (37%) were the most common drug-related toxicities. Skin rashes (>grade 2) correlated with longer OS (7.4 months vs 4.0 (grade 0) or 5.0 months (grade 1)). Recently, a phase I study in SCCHN reported that erlotinib (150 mg qd) in combination with bevacizumab (5, 10 and 15 mg kg−1 i.v. 3q weeks) is feasible and well tolerated in patients with recurrent/metastatic SCCHN (Mauer et al, 2004). In a phase II trial of erlotinib (150 mg p.o.) plus cisplatin/docetaxel in recurrent/metastatic SCCHN, 66% ORR (three CRs, 18 PRs, eight SDs) and 91% DCR were observed. Grade 3/4 toxicities included neutropenia, diarrhoea and rash (Kim et al, 2006).
Lapatinib (TykerbR, GW572016, GlaxoSmithKline, Brentford, London, UK) is a selective kinase inhibitor of both EGFR and HER-2. A phase II trial of lapatinib (1500 mg o.d.) in recurrent/metastastic SCCHN reported no objective responses, suggesting little activity in either EGFR inhibitor naïve or refractory patients (Abidoye et al, 2006) (Table 3).
TKIs in combination with radiotherapy
A phase I/II study combining gefitinib (250 mg p.o. q.d.) with induction chemotherapy (docetaxel/5-FU/carboplatin) in locally advanced SCCHN demonstrated that this regimen was feasible and produced high RR (11 CRs, 18 PRs, five SDs out of 34 patients) (Doss et al, 2006). Two phase I/II studies of erlotinib in combination with docetaxel or cisplatin and radiotherapy in locally advanced SCCHN demonstrated that these combinations are safe and feasible (Herchenhorn et al, 2006; Savvides et al, 2006). Phase II trials are planned or ongoing (Table 4). Recently, results from an ongoing phase I study of lapatinib in combination with cisplatin (100 mg m−2 days 1, 22, 43) plus radiotherapy (66–70 Gy/6–7 weeks) in locally advanced SCCHN demonstrated minor AEs and encouraging clinical activity (Harrington et al, 2006).
Rash and clinical outcome
Acneiform papulopustular skin rash, usually on the face and upper torso, is the most common toxicity found with EGFR antibodies such as cetuximab, panitumumab or matuzumab and the kinase inhibitors gefitinib and erlotinib (Perez-Soler and Saltz, 2005). Rash was mainly grade 1/2, with grade 3 in <13% of patients and no grade 4. Phase I studies indicate that rash is dose dependent. Data from multiple studies with cetuximab, erlotinib and gefitinib show a consistent relationship between rash and response or survival (Cohen et al, 2003, 2005a, 2005b; Soulieres et al, 2004; Baselga et al, 2005; Burtness et al, 2005; Herbst et al, 2005). Little is known about the aetiology of the rash and evidence-based treatment recommendations for rash management are missing owing to the lack of clinical trials addressing this problem (Perez-Soler and Saltz, 2005). Recently, results from a prospective algorithmic approach for the treatment of skin rash in SCCHN patients was presented (Garey et al, 2005) (Table 5). Responses included 11 out of 11 CRs for grade 1, three out of four PRs for grade 2 and one PR for grade 3 rash.
Conclusions and future perspectives
Clinical trials treating SCCHN patients with molecularly targeted treatment strategies designed to specifically inhibit EGFR function have shown promising – albeit limited – levels of efficacy, even as monotherapy. Food and Drug Administration/EMEA approval of cetuximab, in combination with radiotherapy for SCCHN treatment, represents the first new drug registration to treat HNC since methotrexate became available in the 1950s. Cetuximab or TKIs plus radiotherapy have a more favourable toxicity profile when compared to chemoradiotherapy. Multiple phase I/II trials are currently testing combinations of cetuximab or TKIs with chemoradiotherapy in locoregionally advanced SCCHN (Table 4). In recurrent/metastatic SCCHN, cetuximab and three TKIs are currently investigated as monotherapy or in combination with chemotherapy. Owing to high toxicity with cisplatin combinations, taxane regimens may be more feasible. These combination therapies may lead to the use of lower doses of standard chemotherapeutics and thus reduced non-specific toxicity to patients, without loss of anticancer activity. Cisplatin-refractory, recurrent/metastatic SCCHN patients may also benefit from EGFR-targeting strategies.
Interestingly, dual-agent molecular targeting of the EGFR combining cetuximab with TKIs (e.g. gefitinib, erlotinib) enhanced tumour growth inhibition over that observed with either agent alone (Huang et al, 2004; Matar et al, 2004). However, others have found that combination of cetuximab and gefitinib was antagonistic in all cell lines considered, suggesting that a double-hit strategy with Mabs and TKIs must be considered with caution (Fischel et al, 2005). Another strategy may be combination of EGFR antagonists (Mab or TKI) with inhibitors of RAS or phosphatidyl inositol-3 kinase pathways, which are downstream of the EGFR (e.g. PI3K inhibitor LY294002, MEK inhibitor U0126, mevalonate pathway inhibitor lovastatin or farnesyl transferase inhibitor FTI SCH66336). Furthermore, inhibitors of VEGF signalling (e.g. bevacizumab, sorafenib, AZD2171) may be potent partners for novel combination therapies (Table 4). Other potentially interesting targets are EGFR-independent survival pathways (e.g. insulin-like growth factor-1 (IGF-1R)) or GPCRs that mediate their effects through EGFR.
Although anti-EGFR-targeted therapies may lead to PRs and disease stabilisation in some patients, many patients do not benefit from these therapies and responsive cases may eventually develop resistance. Molecular resistance mechanisms include (1) specific EGFR mutations (e.g. EGFRvIII, T790M), (2) constitutive activation of downstream effectors (e.g. loss/inactivation of PTEN, activation of Src, RAS, STAT3/5), (3) increased angiogenesis (upregulation of VEGF) and (4) the presence of redundant tyrosine kinase receptors (e.g. HER-2, c-MET, IGF-1R) (Kobayashi et al, 2005). Many anti-EGFR Mabs are unable to bind the aberrant extracellular domain of EGFRvIII and thus fail to inhibit ligand-induced receptor activation. Interestingly, resistance caused by the T790M mutation can be overcome by CL-387785, a specific and irreversible anilinoquinazoline EGFR inhibitor (Kobayashi et al, 2005). Another major challenge is the development of reliable methods to determine which patient populations are likely to receive the greatest benefit from these novel agents in order to justify the enormous treatment costs of these new drugs.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Abidoye OO, Cohen EE, Wong SJ, Kozloff MF, Nattam SR, Stenson KM, Blair EA, Day S, Dancey JE, Vokes EE (2006) A phase II study of lapatinib (GW572016) in recurrent/metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN). J Clin Oncol 24: 297s (abstract 5568)
Baselga J, Pfister D, Cooper MR, Cohen R, Burtness B, Bos M, D'Andrea G, Seidman A, Norton L, Gunnett K, Falcey J, Anderson V, Waksal H, Mendelsohn J (2000) Phase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. J Clin Oncol 18: 904–914
Baselga J, Trigo JM, Bourhis J, Tortochaux J, Cortes-Funes H, Hitt R, Gascon P, Amellal N, Harstrick A, Eckardt A (2005) Phase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol 23: 5568–5577
Bastholt L, Specht L, Jensen K, Brun E, Loft A, Petersen J, Kastberg H, Eriksen JG (2005) A novel fully human monoclonal antibody against epidermal growth factor receptor (EGFR). First clinical and FDG-PET imaging results from a phase I/II trial conducted by the Danish Head and Neck Cancer Study Group (DAHANCA) in patients with squamous cell carcinoma of the head and neck (SCCHN). Proc Am Soc Clin Oncol 23: 507s (abstract 5530)
Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354: 567–578
Browman GP, Cronin L (1994) Standard chemotherapy in squamous cell head and neck cancer: what we have learned from randomised trials. Semin Oncol 21: 311–319
Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA (2005) Phase III Randomized Trial of Cisplatin Plus Placebo Compared With Cisplatin Plus Cetuximab in Metastatic/Recurrent Head and Neck Cancer: An Eastern Cooperative Oncology Group Study. J Clin Oncol 23: 8646–8654
Cohen EE, Kane MA, List MA, Brockstein BE, Mehrotra B, Huo D, Mauer AM, Pierce C, Dekker A, Vokes EE (2005b) Phase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res 11: 8418–8424
Cohen EE, Lingen MW, Martin LE, Harris PL, Brannigan BW, Haserlat SM, Okimoto RA, Sgroi DC, Dahiya S, Muir B, Clark JR, Rocco JW, Vokes EE, Haber DA, Bell DW (2005a) Response of Some Head and Neck Cancers to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors May Be Linked to Mutation of ERBB2 rather than EGFR. Clin Cancer Res 11: 8105–8108
Cohen EE, Rosen F, Stadler WM, Recant W, Stenson K, Huo D, Vokes EE (2003) Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol 21: 1980–1987
Crombet T, Osorio M, Cruz T, Roca C, del Castillo R, Mon R, Iznaga-Escobar N, Figueredo R, Koropatnick J, Renginfo E, Fernandez E, Alvarez D, Torres O, Ramos M, Leonard I, Perez R, Lage A (2004) Use of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patients. J Clin Oncol 22: 1646–1654
Dassonville O, Formento JL, Francoual M, Ramaioli A, Santini J, Schneider M, Demard F, Milano G (1993) Expression of epidermal growth factor receptor and survival in upper aerodigestive tract cancer. J Clin Oncol 11: 1873–1878
Doss HH, Greco FA, Meluch AA, Gray JR, Spigel DR, Shipley DL, Barton JH, Kuzur ME, Ramsey S, Lunin SD, Hainsworth JD (2006) Induction chemotherapy + gefitinib followed by concurrent chemotherapy/radiation therapy/gefitinib for patients (pts) with locally advanced squamous carcinoma of the head and neck: A phase I/II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol 24: 290s,(abstract 5543)
Fischel JL, Formento P, Milano G (2005) Epidermal growth factor receptor double targeting by a tyrosine kinase inhibitor (Iressa) and a monoclonal antibody (Cetuximab). Impact on cell growth and molecular factors. Br J Cancer 92: 1063–1068
Garey JS, Oishi KJ, Burke B, Kim ES (2005) Treatment of skin rash in a phase II study of cisplatin, docetaxel, and erlotinib in patients with advanced head and neck cancer: a prospective algorithmic approach. J Clin Oncol 23: 786s (abstract 8231)
Harrington KJ, Bourhis J, Nutring CM, Rosine D, Theodosio AM, Gardiner S, Berger MS, Beelen AP, Stead AG, El-Hariry IA (2006) A phase I, open-label study of lapatinib plus chemoradiation in patients with locally advanced squamous cell carcinoma of the head and neck (SCCHN). J Clin Oncol 24: 293s (abstract 5553)
Herbst RS, Arquette M, Shin DM, Dicke K, Vokes EE, Azarnia N, Hong WK, Kies MS (2005) Phase II multicenter study of the epidermal growth factor receptor antibody cetuximab and cisplatin for recurrent and refractory squamous cell carcinoma of the head and neck. J Clin Oncol 23: 5578–5587
Herchenhorn D, Dias FL, Ferreira CG, Bezerra M, Fonseca AJ, Mora P, Pineda M, Fontão K, Knust RE, Martins RG (2006) Phase I/II study of erlotinib combined with cisplatin and radiotherapy for locally advanced squamous cell carcinoma of the head and neck (SCCHN). Proc Am Soc Clin Oncol 24: 298s (abstract 5575)
Huang S, Armstrong EA, Benavente S, Chinnaiyan P, Harari PM (2004) Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res 64: 5355–5362
Ji H, Zhao X, Yuza Y, Shimamura T, Li D, Protopopov A, Jung BL, McNamara K, Xia H, Glatt KA, Thomas RK, Sasaki H, Horner JW, Eck M, Mitchell A, Sun Y, Al-Hashem R, Bronson RT, Rabindran SK, Discafani CM, Maher E, Shapiro GI, Meyerson M, Wong KK (2006) Epidermal growth factor receptor variant III mutations in lung tumorigenesis and sensitivity to tyrosine kinase inhibitors. Proc Natl Acad Sci USA 103: 7817–7822
Kalyankrishna S, Grandis JR (2006) Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol 24: 2666–2672
Kim ES, Kies MS, Glisson BS, Ginsberg LE, Holsinger FC, Burke BJ, Truong M, Tsao AS, Hong WK, Lippman SM (2006) Phase II study of combination cisplatin, docetaxel and erlotinib in patients with metastatic/recurrent head and neck squamous cell carcinoma (HNSCC). J Clin Oncol 24: 285s (abstract 5521)
Kirby AM, A'Hern RP, D'Ambrosio C, Tanay M, Syrigos KN, Rogers SJ, Box C, Eccles SA, Nutting CM, Harrington KJ . (2006) Gefitinib (ZD1839, Iressa) as palliative treatment in recurrent or metastatic head and neck cancer. Br J Cancer 94: 631–636
Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B (2005) EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 352: 786–792
Lee JW, Soung YH, Kim SY, Nam HK, Park WS, Nam SW, Kim MS, Sun DI, Lee YS, Jang JJ, Lee JY, Yoo NJ, Lee SH (2005) Somatic mutations of EGFR gene in squamous cell carcinoma of the head and neck. Clin Cancer Res 2879–2882
Loeffler-Ragg J, Witsch-Baumgartner M, Tzankov A, Hilbe W, Schwentner I, Sprinzl GM, Utermann G, Zwierzina H (2006) Low incidence of mutations in EGFR kinase domain in Caucasian patients with head and neck squamous cell carcinoma. Eur J Cancer 42: 109–111
Matar P, Rojo F, Cassia R, Moreno-Bueno G, Di Cosimo S, Tabernero J, Guzman M, Rodriguez S, Arribas J, Palacios J, Baselga J (2004) Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin Cancer Res 10: 6487–6501
Mauer AM, Cohen EEW, Wong SJ, Kozloff M, Winegarden J, Gustin DM, Vokes EE (2004) Phase I study of epidermal growth factor receptor (EGFR) inhibitor, erlotinib, and vascular endothelial growth factor monoclonal antibody, bevacizumab, in recurrent and/or metastatic squamous cell carcinoma of the head and neck (SCCHN). Proc Am Soc Clin Oncol 22: 497s (abstract 5539)
Morton RP, Rugman F, Dorman EB, Stoney PJ, Wilson JA, McCormick M, Veevers A, Stell PM (1985) Cisplatinum and bleomycin for advanced or recurrent squamous cell carcinoma of the head and neck: a randomised factorial phase III controlled trial. Cancer Chemother Pharmacol 15: 283–289
Moscatello DK, Holgado-Madruga M, Godwin AK, Ramirez G, Gunn G, Zoltick PW, Biegel JA, Hayes RL, Wong AJ (1995) Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Res 55: 5536–5539
Mrhalova M, Plzak J, Betka J, Kodet R (2005) Epidermal growth factor receptor–its expression and copy numbers of EGFR gene in patients with head and neck squamous cell carcinomas. Neoplasma 52: 338–343
Perez-Soler R, Saltz L (2005) Cutaneous adverse effects with HERl/EGFR-targeted agents: is there a silver lining? J Clin Oncol 23: 5235–5246
Pfister DG, Su YB, Kraus DH, Wolden SL, Lis E, Aliff TB, Zahalsky AJ, Lake S, Needle MN, Shaha AR, Shah JP, Zelefsky MJ (2006) Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: a pilot phase II study of a new combined-modality paradigm. J Clin Oncol 24: 1072–1078
Pignon JP, Bourhis J, Domenge C, Designe L (2000) Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet 355: 949–955
Robert F, Ezekiel MP, Spencer SA, Meredith RF, Bonner JA, Khazaeli MB, Saleh MN, Carey D, LoBuglio AF, Wheeler RH, Cooper MR, Waksal HW (2001) Phase I study of anti--epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol 19: 3234–3243
Rogers SJ, Harrington KJ, Rhys-Evans P, O-Charoenrat P, Eccles SA (2005) Biological significance of c-erbB family oncogenes in head and neck cancer. Cancer Metastasis Rev 24: 47–69
Santini J, Formento JL, Francoual M, Milano G, Schneider M, Dassonville O, Demard F (1991) Characterization, quantification, and potential clinical value of the epidermal growth factor receptor in head and neck squamous cell carcinomas. Head Neck 13: 132–139
Savvides P, Agarwala SS, Greskovich J, Argiris A, Bokar J, Cooney M, Hoppel C, Stepnick DW, Lavertu P, Remick S (2006) Phase I study of the EGFR tyrosine kinase inhibitor erlotinib in combination with docetaxel and radiation in locally advanced squamous cell cancer of the head and neck (SCCHN). J Clin Oncol 24: 291s (abstract 5545)
Shin DM, Donato NJ, Perez-Soler R, Shin HJ, Wu JY, Zhang P, Lawhorn K, Khuri FR, Glisson BS, Myers J, Clayman G, Pfister D, Falcey J, Waksal H, Mendelsohn J, Hong WK (2001) Epidermal growth factor receptor-targeted therapy with C225 and cisplatin in patients with head and neck cancer. Clin Cancer Res 7: 1204–1213
Sok JC, Coppelli FM, Thomas SM, Lango MN, Xi S, Hunt JL, Freilino ML, Graner MW, Wikstrand CJ, Bigner DD, Gooding WE, Furnari FB, Grandis JR (2006) Mutant Epidermal Growth Factor Receptor (EGFRvIII) Contributes to Head and Neck Cancer Growth and Resistance to EGFR Targeting. Clin Cancer Res 12: 5064–5073
Sonnweber B, Dlaska M, Skvortsov S, Dirnhofer S, Schmid T, Hilbe W (2006) High predictive value of epidermal growth factor receptor phosphorylation but not of EGFRvIII mutation in resected stage I non-small cell lung cancer (NSCLC). J Clin Pathol 59: 255–259
Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL (2004) Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 22: 77–85
Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, Knecht R, Amellal N, Bessa EH, Baselga J, Vermorken JB (2004) Cetuximab monotherapy is active in patients (pts) with platinum-refractory recurrent/metastatic squamous cell carcinoma of the head and neck (SCCHN): Results of a phase II study. Proc Am Soc Clin Oncol 22: 488s (abstract 5502)
Wheeler RH, Jones D, Sharma P, Davis RK, Spilker H, Boucher K, Leachman S, Grossman D, Salzman K, Akerley W (2005) Clinical and molecular phase II study of gefitinib in patients (pts) with recurrent squamous cell cancer of the head and neck (H&N Ca). Proc Am Soc Clin Oncol 24: 507s (abstract 5531)
Wirth LJ, Haddad RI, Lindeman NI, Zhao X, Lee JC, Joshi VA, Norris Jr CM, Posner MR (2005) Phase I study of gefitinib plus celecoxib in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol 23: 6976–6981
Acknowledgements
This work was supported in part by grants to CR from the Deutsche Krebshilfe (10-1801-Re-I), Hannover Medical School (HiLF program) and the Deutsche José Carreras Leukämie-Stiftung e.V. (DJCLS R05/21). This work was also supported by grants to MM from the International Myeloma Foundation (IMF), the Hannover Medical School (HiLF program), and the Dr Hiltrud-Pulst Myelom Fund. We apologize to our colleagues for the omission of many relevant citations owing to space constraints.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Reuter, C., Morgan, M. & Eckardt, A. Targeting EGF-receptor-signalling in squamous cell carcinomas of the head and neck. Br J Cancer 96, 408–416 (2007). https://doi.org/10.1038/sj.bjc.6603566
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6603566
Keywords
This article is cited by
-
The effect of metabolic syndrome on head and neck cancer incidence risk: a population-based prospective cohort study
Cancer & Metabolism (2021)
-
Epidermal Growth Factor Receptor’s Function in Cutaneous Squamous Cell Carcinoma and Its Role as a Therapeutic Target in the Age of Immunotherapies
Current Treatment Options in Oncology (2020)
-
NIPA-like domain containing 1 is a novel tumor-promoting factor in oral squamous cell carcinoma
Journal of Cancer Research and Clinical Oncology (2018)
-
Visualizing epithelial expression of EGFR in vivo with distal scanning side-viewing confocal endomicroscope
Scientific Reports (2016)
-
EGFR Overexpressed in Colonic Neoplasia Can be Detected on Wide-Field Endoscopic Imaging
Clinical and Translational Gastroenterology (2015)