Abstract
In a multivariate analysis of 154 patients receiving chemotherapy, baseline CA19-9 was an independent prognostic factor for overall survival (OS) (HR 1.8; 95% CI: 1.3–2.5, P=0.0004). The 1-year OS was 19 and 46%, respectively, for patients with a baseline CA19-9 above or below the median value. A fall of 20% in CA19-9 level from baseline was an independent prognostic factor for OS (HR 1.9; 95% CI: 1.1–3.4, P=0.019).
Similar content being viewed by others
Main
Over the last decade, systemic chemotherapy has become a standard therapy for patients with inoperable pancreatic cancer. Prior to this, the lack of randomised data and the perceived toxicity of treatment led most clinicians to believe that cytotoxic therapy would not be of palliative benefit or could significantly prolong their patients' lives. Following a number of randomised clinical trials, it became clear that not only did chemotherapy offer a small but significant survival benefit, but there was also evidence supporting an improvement in quality of life. Over the last decade, a number of relatively large and well-conducted phase III trials have attempted to define the optimal chemotherapy schedule for this cohort of patients. Currently, the standard of care is gemcitabine, following the seminal paper by Burris et al (1997). A number of ongoing trials are exploring the potential benefit of gemcitabine scheduling, combination with other chemotherapeutic agents and the addition of novel agents such as antibodies to the epidermal growth factor receptor and vascular endothelial growth factor.
In the mid 1990s, the CONSORT (Consolidated Standards of Reporting Trials) statement was published, which was intended to improve the quality of reporting of phase III trials (Moher et al, 2001). The CONSORT statement consists of a checklist and a diagram intended for use in writing, reviewing or assessing reports of randomised controlled trials. One of the items in the checklist is the reporting of baseline demographic and clinical characteristics of each group.
When assessing the results of a phase III trial, it is clearly vital that the known important prognostic factors are balanced between treatment arms to allow a fair comparison of treatment effect. Despite a small number of publications suggesting that the tumour marker CA19-9 is an important prognostic variable (Gogas et al, 1998; Halm et al, 2000; Saad et al, 2002; Stemmler et al, 2003; Ziske et al, 2003), none of the large published randomised studies of chemotherapy to date have reported baseline CA19-9 levels.
The aim of this retrospective analysis was to investigate whether CA19-9 provided prognostic information in patients with inoperable pancreatic cancer treated with systemic chemotherapy, and should therefore be reported in future randomised controlled trials.
Materials and methods
All patients treated with gemcitabine- and/or 5-fluorouracil-based chemotherapy in three consecutive phase III randomised trials at this centre were eligible for analysis. Patients had inoperable or metastatic histologically proven pancreatic cancer, were of WHO performance status (PS) 0–2 and had radiologically measurable disease. All patients were previously untreated. Entry criteria included a total serum bilirubin level of 30 μmol l−1 or less. Informed written consent was obtained from all patients.
Treatment
All eligible patients received one of four chemotherapy regimens. Protracted venous infused 5-fluorouracil (PVI 5FU) was either given alone (at a dose of 300 mg m−2 day−1 for a maximum of 24 weeks) or in combination with mitomycin C (MMC) administered at a dose of 7 mg m−2 6 weekly for four courses. The remainder received a combination of gemcitabine (1000 mg m−2 on days 1, 8 and 15 of a 28-day cycle) and capecitabine (830 mg m−2 twice daily for 21 days followed by a week's break) or gemcitabine alone, at a dose of 1000 mg m−2 weekly for 7 weeks, followed by a week's break and subsequently for 3 weeks out of every 4.
Measurements
Serum CA19-9 was measured at baseline (defined as being within 30 days before the start of chemotherapy) and subsequently every 6 weeks while the patients remained on treatment. The CA19-9 concentration was measured by an automated, commercially available enzyme immunoassay on an axsym analyser (Abbott Diagnostics Laboratory). A value of 37 U ml−1 was used as the upper limit of normal.
Statistics
All data were recorded prospectively on the Gl unit database. Overall survival (OS) (defined as time from randomisation to death from any cause) was examined with the Kaplan–Meier product limit method (Kaplan and Meier, 1958), and survival in patients with baseline CA19-9 values that fell above or below the median level (median dichotomised) was compared with the log-rank test (Peto and Peto, 1972). Statistical analysis was performed using the statistical package SPSS for Windows version 12.01. Multivariate logistic regression was used to determine factors predictive of survival. Factors included in these analyses were baseline CA19-9, a 20% drop in CA19-9 level following the start of chemotherapy, age, sex, the presence of locally advanced or metastatic disease and performance status. A fall of 20% in the CA19-9 level was chosen after review of similar analyses in the literature. P-values of less than 0.05 were considered statistically significant.
Results
Patients
Between July 1994 and July 2004, 218 patients received chemotherapy in the context of a clinical trial. Of these patients, 154 had a CA19-9 level recorded within 30 days before the start of chemotherapy at this centre. Baseline demographics are given in Table 1.
CA19-9 levels
Baseline CA19-9 levels were recorded in 154 patients. The median time of performing the test was 1 day prior to the start of chemotherapy (range 0–30). The median baseline CA19-9 level was 958 U ml−1.
Post-treatment CA19-9 was recorded in 88 patients. The median time of performing the test from the start of chemotherapy was 42 days, ranging from 28 to 55 days. The median post-treatment CA19-9 level was 998 U ml−1. There was no significant difference in the time to progression (TTP) between the cohort that had CA19-9 levels performed at baseline and following chemotherapy, compared with the cohort that had baseline levels only (P=0.46).
Survival
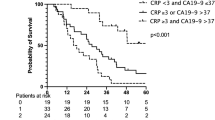
The median follow-up in those patients with a recorded baseline CA19-9 level was 712 days and 92% had died at the time of analysis. The median OS in patients with a baseline CA19-9 below the median value was significantly superior to those with a value above (337 vs 165 days, P=0.0004). The 1-year OS was 19 and 46%, respectively, for patients with a baseline CA19-9 above or below the median value (see Figure 1).
In a multivariate analysis, baseline CA19-9 above or below the median value (958 U ml−1) was found to be an independent prognostic factor for OS (HR 1.8; 95% CI: 1.3–2.5, P=0.0004).
A fall of 20% in CA19-9 level at first measurement following the start of treatment was an independent prognostic factor for OS (HR 1.9; 95% CI: 1.1–3.4, P=0.019). Baseline characteristics in this group were not significantly different from the original cohort.
Other factors that were found to be independent prognostic factors for OS were sex and performance status (PS) (see Table 2).
Discussion
Ensuring an equal balance of prognostic factors between treatment arms in randomised controlled trials is vital. This may be particularly relevant when the clinical benefit of an intervention is likely to be small, as is the case for patients with advanced pancreatic cancer. A number of both patient- and disease-related factors have been identified as independent prognostic markers, including PS, sex and the presence of metastases, and are routinely quoted in clinical studies as baseline characteristics. There have also been several reports of various biological markers that may carry some prognostic significance. These include growth factors such as vascular endothelial growth factor and platelet-derived endothelial cell growth factor (Fujimoto et al, 1998), microsatellite instability (Nakata et al, 2002), tumour suppression gene expression such as SMAD4 (Biankin et al, 2002) and the expression of mutated genes controlling response to DNA damage, such as GADD45a and p53 (Yamasawa et al, 2002).
The serum carbohydrate antigen CA19-9 is a tumour-associated antigen that has been shown to be a highly specific and sensitive serum marker for pancreatic cancer (Pleskow et al, 1989; Rollhauser and Steinberg, 1998). CA19-9 is a sialylated Lewis blood group antigen targeted by the monoclonal antibody 1116 NS 19-9 (Koprowski et al, 1979). Previous studies have demonstrated a prognostic role for pretreatment CA19-9 in patients receiving radiotherapy (Katz et al, 1998) or undergoing pancreatic resection (Glenn et al, 1988). Although a small number of papers have described a fall in CA19-9 to be an independent prognostic variable for survival (Gogas et al, 1998; Saad et al, 2002; Stemmler et al, 2003; Ziske et al, 2003), there have been surprisingly few studies investigating the use of baseline CA19-9 in predicting survival for patients with inoperable pancreatic cancer who undergo systemic chemotherapy. Halm et al (2000) reported the survival of 43 consecutive patients with advanced pancreatic cancer treated with single-agent gemcitabine. In a multivariate analysis, baseline CA19-9 was reported to be an independent prognostic predictor of survival with a relative risk of death 1.4 (95% CI: 1.0–2.0, P=0.04). Ziske et al (2003) published their results in a similar cohort of patients (n=46). Although they did not report baseline CA19-9 level as a prognostic variable, they did find that a fall of at least 20% in CA19-9 level following the start of chemotherapy was the only independent prognostic marker for OS in their Cox multivariate analysis. An important caveat in the interpretation of CA19-9 levels in pancreatic cancer is the presence of biliary obstruction, which results in the elevation of the marker level. In the current series, however, entry criteria mandated that serum bilirubin should be 30 μmol l−1 or less, and therefore this potential pitfall was avoided in the interpretation of baseline CA19-9 levels.
The results of this study suggest that baseline tumour marker CA19-9 is an important independent prognostic variable in patients with inoperable pancreatic cancer. We found that baseline CA19-9 above or below the median value (958 U ml−1) was an independent prognostic factor for OS (HR 1.8; 95% CI: 1.3–2.5, P=0.0004), with a 1-year OS of 19 and 46%, respectively. Although this is the largest reported series examining the predictive value of baseline CA19-9 in patients treated with systemic chemotherapy, it is still a relatively small cohort of 154 patients, and further studies would be welcome to consolidate these results. However, considering its apparent prognostic value, future randomised controlled trials should consider reporting median CA19-9 levels in treatment groups as part of routine demographic data.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Biankin AV, Morey AL, Lee CS, Kench JG, Biankin SA, Hook HC, Head DR, Hugh TB, Sutherland RL, Henshall SM (2002) DPC4/Smad4 expression and outcome in pancreatic ductal adenocarcinoma. J Clin Oncol 20: 4531–4542
Burris 3rd HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Stomiolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15: 2403–2413
Fujimoto K, Hosotani R, Wada M, Lee JU, Koshiba T, Miyamoto Y, Tsuji S, Nakajima S, Doi R, Imamura M (1998) Expression of two angiogenic factors, vascular endothelial growth factor and platelet-derived endothelial cell growth factor in human pancreatic cancer, and its relationship to angiogenesis. Eur J Cancer 34: 1439–1447
Glenn J, Steinberg WM, Kurtzman SH, Steinberg SM, Sindelar WF (1988) Evaluation of the utility of a radioimmunoassay for serum CA 19-9 levels in patients before and after treatment of carcinoma of the pancreas. J Clin Oncol 6: 462–468
Gogas H, Lofts FJ, Evans TR, Daryanani S, Mansi JL (1998) Are serial measurements of CA19-9 useful in predicting response to chemotherapy in patients with inoperable adenocarcinoma of the pancreas? Br J Cancer 77: 325–328
Halm U, Schumann T, Schiefke I, Witzigmann H, Mossner J, Keim V (2000) Decrease of CA 19-9 during chemotherapy with gemcitabine predicts survival time in patients with advanced pancreatic cancer. Br J Cancer 82: 1013–1016
Kaplan EL, Meier P (1958) Non parametric estimation from incomplete observations. J Am Stat Assoc 53: 457–481
Katz A, Hanlon A, Lanciano R, Hoffman J, Coia L (1998) Prognostic value of CA 19-9 levels in patients with carcinoma of the pancreas treated with radiotherapy. Int J Radiat Oncol Biol Phys 41: 393–396
Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Heriyn D, Fulner P (1979) Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet 5: 957–971
Moher D, Schulz KF, Altman DG (2001) The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 357: 1191–1194
Nakata B, Wang YQ, Yashiro M, Nishioka N, Tanaka H, Ohira M, Ishikawa T, Nishino H, Hirakawa K (2002) Prognostic value of microsatellite instability in resectable pancreatic cancer. Clin Cancer Res 8: 2536–2540
Peto R, Peto J (1972) Asymtolically efficient invariant procedures. J R Stat Soc A135: 185–206
Pleskow DK, Berger HJ, Gyves J, Allen E, McLean A, Podolsky DK (1989) Evaluation of a serologic marker, CA19-9, in the diagnosis of pancreatic cancer. Ann Intern Med 110: 704–709
Rollhauser C, Steinberg W (1998) Tumor antigens in pancreatic cancer. In Pancreatic Cancer Reber HA (ed) pp 137–156. Totowa, NJ: Humana Press
Saad ED, Machado MC, Wajsbrot D, Abramoff R, Hoff PM, Tabacof J, Katz A, Simon SD, Gansl RC (2002) Pretreatment CA 19-9 level as a prognostic factor in patients with advanced pancreatic cancer treated with gemcitabine. Int J Gastrointest Cancer 32: 35–41
Stemmler J, Stieber P, Szymala AM, Schalhom A, Schermuly MM, Wilkowski R, Helmberger T, Lamerz R, Stoffregen C, Niebler K, Garbrecht M, Heinemann V (2003) Are serial CA 19-9 kinetics helpful in predicting survival in patients with advanced or metastatic pancreatic cancer treated with gemcitabine and cisplatin? Onkologie 26: 462–467
Yamasawa K, Nio Y, Dong M, Yamaguchi K, Itakura M (2002) Clinicopathological significance of abnormalities in Gadd45 expression and its relationship to p53 in human pancreatic cancer. Clin Cancer Res 8: 2563–2569
Ziske C, Schlie C, Gorschluter M, Glasmacher A, Mey U, Strehl J, Sauerbruch T, Schmidt-Wolf IG (2003) Prognostic value of CA 19-9 levels in patients with inoperable adenocarcinoma of the pancreas treated with gemcitabine. Br J Cancer 89: 1413–1417
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Maisey, N., Norman, A., Hill, A. et al. CA19-9 as a prognostic factor in inoperable pancreatic cancer: the implication for clinical trials. Br J Cancer 93, 740–743 (2005). https://doi.org/10.1038/sj.bjc.6602760
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6602760
Keywords
This article is cited by
-
Diagnosing and monitoring pancreatic cancer through cell-free DNA methylation: progress and prospects
Biomarker Research (2023)
-
Circulating NPTX2 methylation as a non-invasive biomarker for prognosis and monitoring of metastatic pancreatic cancer
Clinical Epigenetics (2023)
-
[18F] AlF-NOTA-FAPI-04 PET/CT can predict treatment response and survival in patients receiving chemotherapy for inoperable pancreatic ductal adenocarcinoma
European Journal of Nuclear Medicine and Molecular Imaging (2023)
-
Liquid Biopsy in Low-Grade Glioma: A Systematic Review and a Proposal for a Clinical Utility Score
Cellular and Molecular Neurobiology (2023)
-
Beta-blockers have no impact on survival in pancreatic ductal adenocarcinoma prior to cancer diagnosis
Scientific Reports (2021)