Abstract
The Controlling Nutritional Status (CONUT) score is a marker of nutrition and is associated with poor survival in various kinds of cancers. However, no reports have yet compared risk factors for colorectal cancer recurrence using a nutritional index. We assessed the predictive value of the CONUT score compared with the modified Glasgow Prognostic Score (mGPS) and Prognostic Nutritional Index (PNI) in colorectal cancer (CRC) patients. We performed a retrospective cohort study of the medical records of 336 consecutive patients with stage I-I I I CRC who underwent curative resection at a single institution in 2012–2017. Univariate and multivariate analyses were conducted to identify prognostic factors associated with relapse-free survival (RFS) and overall survival (OS). The low CONUT score group exhibited higher RFS and longer OS compared to the high CONUT score group (82.2% vs. 63.3%, p = 0.002 and 95.5% and 86.2%, p = 0.005, respectively). The Akaike’s information criterion values of each index for RFS and OS were superior in CONUT score (723.71 and 315.46, respectively) compared to those of PNI (726.95 and 316.52) and mGPS (728.15 and 318.07, respectively). The CONUT score was found to be a good predictor of RFS and OS in patients with resectable CRC.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC), one of the most common and aggressive malignancies, is the fourth-leading cause of cancer-related death worldwide1. The Controlling Nutritional Status (CONUT) score—which is comprised of the serum values of albumin (ALB) , total lymphocyte count (TLC), and total cholesterol—is considered a potential predictor of poor outcomes in several types of malignances, including colorectal cancer2,3. Two nutritional indicators, i.e., the modified Glasgow Prognostic Score (mGPS) and the Prognostic Nutritional Index (PNI), have been widely established predictors of the prognoses of patients with cancer4,5,6.
We, clinicians, need tools that are simpler and more straightforward than complex diagnostic and prognostic tools. A variety of laboratory biomarkers have been developed that use the systemic inflammation or nutritonal status of patients as a simple tool. These biomarkers have been demonstrated to predict the prognosis in various cancers, and several studies have demonstrated that these indicators are predictive of the prognosis of patients with CRC2,7,8. However, to the best of our knowledge, there is no report on which prognostic indicators are the most useful for CRC patients after curative surgery. We conducted the present study to determine the utility of these predictive indicators, based on nutritional factors, in CRC patients after their curative surgery.
Results
Patient characteristics
The median age of the 301 patients was 67 (range, 22–93) years; 180 (60.1%) patients were male and 121 (39.9%) were female. Primary tumors were located in the right of the colon in 91 cases (30.2%) and in the left side of the colon or rectum in 210 (69.8%). Histologically, 278 tumors (89.0%) were moderately or well differentiated, and the other 33 (11.0%) were not (Table 1). Seventy-seven (25.6%) early complications were noted; these comprised surgical site infections (n = 20), ileus (n = 10), anastomosis leakage (n = 8), delirium (n = 7), respiratory infections (n = 6), urinary disturbances (n = 5), urinary infections (n = 5), colitis (n = 3), hemorrhage (n = 6), and complications (n = 8).
Determination of cut-off values
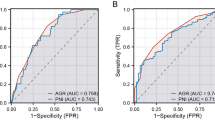
The ROC curve analysis results indicated that the most appropriate cutoff value for the CONUT score was 3. All patients were categorized into the high CONUT score group (score ≥ 3; n = 106, 35.2%) or low CONUT score group (score = 0 or 1 or 2; n = 195, 64.8%).
ALB, TLC, cholesterol scattergraph by CONUT score
The mean values of the low CONUT score group were as follows. ALB: 4.1, TLC: 1,641, and cholesterol: 195, and those of the high CONUT score group were 3.4, 1,101, and 159 (Fig. 1). All items were significantly different between the two groups. None of the patients in the low-cholesterol group was taking cholesterol medication.
Prognostic factors
We used a univariate analysis and the Cox regression model to identify risk factors for recurrence after surgery. It is difficult to evaluate the nutritional indices together as prognostic factors because there is strong confounding among them. We examined each nutritional index and clinicopathological factors. A CONUT score ≥ 3 lymphatic invasion, vascular invasion, pT category, preoperative CEA level, and early complications were significantly associated with poor RFS in the univariate survival analyses (Table 2). Other factors including sex, age, tumor location, preoperative CEA, histological grade, and mucinous or signet ring cell type were not significantly associated with RFS. The multivariate analysis identified a CONUT score ≥ 3 status as an independent prognostic factor associated with RFS (Table 2).
The results of the univariate and multivariate analyses for OS are summarized in Table 3. For the univariate analyses, vascular invasion, lymph invasion, pT category, N category, preoperative CEA level, CONUT score, and PNI were significantly associated with the patients' OS. In the multivariate analyses for OS, the CONUT score and lymph invasion were independent predictive factors.
Relationships between clinicopathologic features and CONUT score status
The CONUT score was significantly associated with age (p = 0.002), pT category (p = 0.002), and early complications (p = 0.031) (Table 4).
Relapse-free survival and overall survival
Of the total of 301 patients with a median follow-up of 1,392 days (interquartile range, 22–2,352 days) developed disease recurrence 70 (23.3%). Among the 70 patients with recurrence, liver metastases were observed in 21 (30%), lung metastases in 14 (20%), local recurrence in 11 (15.7%), para-aortic lymph nodes in 6 (8.5%), liver and lung metastases in 7 (10%), peritoneal carcinomatosis in 5 (7.1%), others in 6 (8.6%). As illustrated in Fig. 2, the low CONUT score group exhibited a significantly higher 3-year RFS rate and significantly longer OS compared to the high CONUT score group: RFS = 82.2% versus 63.3% (HR 2.14, 95%CI: 1.33–3.42, p = 0.002 Fig. 2), and the 3-year OS rates were 95.5% and 86.2% (HR 1.98, 95%CI: 1.27–3.83, p = 0.005, Fig. 2).
As illustrated in Fig. 3, the 3-year RFS rate was 78.6% in the low mGPS group and 67.4% in the high mGPS group (HR 1.83, 95%CI: 1.02–3.29, p = 0.042), with a significant between-group difference (Fig. 4, HR 2.18, 95%CI: 1.45–4.87, p = 0.002). In addition, the 3-year RFS was significantly lower at 61.1% in the low PNI group compared to 79.9% in the high PNI group (HR 1.83, 95%CI: 1.02–3.29, p = 0.042, Fig. 3). The 3-year OS was 95.2% in the low mGPS group and 83.1% in the high mGPS group (Fig. 3, p = 0.06), with no significant difference between the low and high mGPS groups. However, the 3-year OS was significantly lower at 84.8% in the low PNI group compared to 94.5% in the high PNI group (HR 2.88, 95%CI: 1.18–7.04, p = 0.020, Fig. 3).
AIC (Akaike's information criterion) of each index model
The AIC values of each index for RFS were 723.713 for the CONUT score, 726.948 for the PNI, 728.146 for the mGPS. According to this comparison, the CONUT score had the best goodness-of-fit, followed by the PNI and mGPS. In OS, the AIC values of each index for RFS were 315.459 for the CONUT score, 316.516 for the PNI, 318.074 for the mGPS; thus, the CONUT score had the best goodness-of-fit, followed by the PNI and mGPS.
Correlation between albumin, lymphocyte count and CRP
The correlation between albumin concentrations and CRP and the correlation between albumin concentrations and TLC were compared. As a result, CRP was R = 0.436, TLC was R = 0.15579, and CRP was more correlated with albumin concentrations (Fig. 4).
Discussion
We evaluated and compared the prognostic impacts of the CONUT score, the PNI, and the mGPS in 301 patients with colorectal cancer, and our analyses revealed that only the CONUT score was an independent prognostic factor for RFS and OS, and it was superior to the other nutritional-based markers in terms of predictive ability for prognosis before initial treatment.
The CONUT score is known as a nutritional assessment index. Recently, the CONUT score has been reported to help estimate postoperative recurrence rates and predict the prognosis of patients with gastrointestinal (GI) cancer. The CONUT score is composed of three common factors: the serum albumin level, which is a nutritional index, the peripheral lymphocyte count, and the total cholesterol concentration. Serum albumin has been reported to correlate with tumor necrosis because inflammatory cytokines reduce albumin synthesis9. As a result, hypoalbuminemia is reported to be a risk factor for the increased prevalence of high-grade colorectal cancer10. Peripheral lymphocytes, which play an important role in the immune response to tumors, are known to indicate an individual's immunological and nutritional status11. We reported that an elevated neutrophil-to-lymphocyte ratio on postoperative day 7 is a significant independent predictor of reduced RFS for patients with stage II colorectal cancer12. Lymphocytes, the main cells of the body's immunity, make an immune response to tumor cells, so a reduction in lymphocytes leads to a reduced ability to block tumors in the body. Therefore, high NLR levels are effective for tumor survival and lead to poor prognosis. Hypercoagulability of blood is leading for metastasis of malignant tumors13,14. The total cholesterol concentration is known as an indicator of a patient's reserve calories15. Other studies indicated that low serum cholesterol levels were associated with a poorer prognosis in patients with various cancers16,17,18. It remains unclear why low serum cholesterol levels are associated with a poor prognosis. The total cholesterol levels have been reported to correlate with tumor progression as tumor tissue reduces the body’s plasma cholesterol levels and caloric intake19. Therefore, hypocholesterolemia is not considered to be the cause of cancer but is thought to be caused by cancer18,20.
We observed herein that the CONUT score was associated with age, pT category, and early complications. Elderly patients often have poor nutritional status compared to younger people. The number of early complications after surgery may have been affected by our patients' poor nutritional status.
The PNI, which is the nutritional index calculated using the peripheral lymphocyte count and the serum albumin level, was reported to be associated with the survival of CRC patients21. The GPS has been used in several different oncological field and could, due to the routine assessment of albumin and CRP, be assessed in all aspects of the prospective care of patients with cancer22,23. In the present study, although the PNI and the CONUT score were found to be predictive factors for RFS and OS in the univariate analyses, the results of the multivariate analysis demonstrated that only the CONUT score was an independent prognostic factor for these patients with CRC.
In addition, the CONUT score more accurately predicted the survival of the CRC patients than the PNI and the mGPS. Although the CONUT score. PNI, and mGPS have common factors for nutrition, they led to different results. We thus investigated the reasons why the CONUT score was superior to the PNI and mGPS in predicting the patients' prognoses. Notably, an additional factor evaluated in the CONUT score which is not included in the mGPS or the PNI is the total cholesterol concentrations. The mGPS is composed of albumin and CRP, and PNI is composed of serum albumin concentrations and TLC. Serum albumin concentrations was included in all scores, and the correlation between albumin and TLC and CRP was lower in TLC (Fig. 4). It may be diagnosing a factor associated with recurrence that is different from serum albumin concentrations. It was considered that the diagnostic value increased because the cholesterol variable was further increased in the group separated by serum albumin concentrations and TLC.
Our study is that post operative early comlications were risk factors of recurrence. It has been reported that the risk of recurrence increases with anastomosis leakage, which is one of the postoperative complications24. There are also reports that perioperative blood transfusion blood and subsequent development of postoperative infectious complications may be associated with a poor prognosis25. In our study, 8 cases of anastomosis leakage and 7 cases of early postoperative complications accompanied by blood transfusion were considered to have influenced this.
This study has its limitations. First, this study was retrospective in design and included patients from a single institution. Secondly, patients underwent a variety of invasive surgical procedures that resulted in higher mortality and morbidity, which this study did not consider. Third, there is no consensus regarding the CONUT score cut-off value, and this makes it difficult to use the CONUT score in clinical settings. We selected the CONUT cut-off value herein by performing an ROC analysis. The CONUT score is a non-specific marker of nutrition, which implies that another systemic disease can affect the CONUT score. Our findings need further review and validation in more CRC patients.
Conclusion
Pretreatment CONUT score in CRC patients was demonstrated to be an independent predictor of RFS and OS. It was superior to PNI and mGPS in predicting the prognosis of patients who underwent curative surgery for CRC. The cost of measuring the CONUT score is very low and can easily be obtained using laboratory data from routine clinical practice. We recommend that patients calculate their CONUT score regularly before undergoing curative surgery. This could be a useful indicator for prognosis in CRC.
Patients and methods
Patient selection
We enrolled 301 consecutive patients with stage I–III CRC diagnosed based on the 8th edition of the American Joint Committee on Cancer (AJCC) staging system26 and treated with curative resection at Teikyo University Hospital, Japan during the period 2012–2017. The surgery of all of the patients was elective. This retroprospective study was approved by the ethics of Teikyo University (Registation Number; 16-032). Informed consent was obtained from all participants, the reporting of our research is in accordance with the STROBE guidelines27.
The variables assessed included patient age, sex, tumor location, histological grade, TNM (tumor, nodes, metastasis) stage, personal medical history, preoperative laboratory data, early complications, and follow-up status. The CONUT score and the PNI were calculated on based on the patient's preoperative laboratory data using. Early complications included anastomotic leakage surgical site infection, colitis, ileus, urinary tract infection, respiratory infection, and dysuria.
The following patients were excluded: (a) those who received adjuvant chemotherapy, (b) those with multiple primary malignancies , and (c) those with familial adenomatous polyposis or Lynch syndromes. Histopathological,clinical and laboratory data were obtained from the patients and blood sampling test was conducted within 1–2 weeks prior to the surgery. Total of 35 patients (neoadjuvant therapy 7, adenomatous polyposis 2, multiple primary malignancies 5 and data not available 21) were excluded (Fig. 5).
CONUT score
Each patient's CONUT score was calculated using his/her data of serum albumin, total peripheral lymphocyte count, and total cholesterol concentrations, based on a previous report that used preoperative serum samples28. Albumin concentrations ≥ 3.5, 3.5 > and > 3.0, 2.99 > and ≥ 2.5, and < 2.5 g/dL were scored as 0, 2, 4, and 6 points, respectively; (2) total lymphocyte count ≥ 1,600, 1599–1,200, 1,199–800, and < 800/mm3 were scored as 0, 1, 2, and 3 points, respectively; and (3) total cholesterol concentrations ≥ 180, 140–179, 100–139, and < 100 mg/dL were scored as 0, 1, 2, and 3 points, respectively. The CONUT score was defined as the sum of items (1), (2), and (3). The CONUT score thus ranges from 0 to 12, with higher scores indicating a worse nutritional status.
mGPS score
The GPS score is as follows: hypoalbuminemia (< 35 g/L) and the elevated C-reactive protein (CRP) (> 10 mg/L) was given a score of 2. Hypoalbuminemia (< 35 g/L) or CRP > 10 mg/L was score of 1. Albumin concentrations ≥ 3.5 g/dL and CRP ≤ 10 mg/L was score of 09. However, hypoalbuminemia alone was not associated with reduced survival, so mGPS was created. The mGPS score 2 is hypoalbuminemia (< 35 g/L) and the elevated CRP (> 10 mg/L), score 1 was the elevated CRP (> 10 mg/L), score 0 is hypoalbuminemia (< 35 g/L) or serum albumin level (> 35 g/L) and the serum CRP lebel (< 10 mg/L)29.
PNI
The PNI, which is calculated using serum albumin and the peripheral lymphocyte count, is a simple and useful score for predicting the prognosis of patients with various cancers30. The PNI was calculated using the following formula: PNI = serum albumin level (g/dL) + 5 × total lymphocyte count21. Onodera reported that this index provided an accurate, quantitative estimate of operative risk31. In general, resection and anastomosis of the gastrointestinal tract can be safely practiced when the index is > 45. The same procedure may be dangerous when the index is between 45 and 40. When the PNI is < 40, this surgery may be contraindicated31. In other studies, the cutoff value of PNI was 40, which is common, We thus set the cut-off value for the PNI as 4021,32,33.
AIC (Akaike information criterion)
The AIC is a popular method for comparing the adequacy of multiple, possibly nonnested models34. A statistic that evaluates the predictability of a statistical model using the difference between the observed value and the theoretical value. The smaller the value, the better the fit35.
Follow-up
Surgical resection was defined as radical when there was no evidence of the tumor clearance and distant metastasis was both histologically and macroscopically complete. The patients were followed up every 3 months for the first 3 years, every 6 months for the next 2 years, and once annually thereafter. At each follow-up, all of the patients underwent a physical examination as well as the measurement of serum carcinoembryonic antigen (CEA) and CA19-9 (carbohydrate antigen 19-9). They also underwent full colonoscopies at 1–2 years after the surgery (retum cancer was every 1 year after surgery). Chest-abdominal computed tomography scans were generally obtained every 6 months. Recurrence was defined as the emergence of a radiological, clinical, and/or pathological diagnosis of cancer cells locally or distant from the original position.
Statistical analysis
Relapse-free survival (RFS) and overall survival (OS) were calculated from the date of the patient underwent surgery to that of recurrence or death, using the Kaplan–Meier method. A Cox regression analysis was performed to identify factors that are significantly associated with RFS or OS. Probability (p)-values ≤ 0.05 were considered significant. The Pearson product-moment correlation coefficient was used for the bivariate correlation. All statistical analyses were performed using JMP 14 software (SAS, Cary, NC, USA).
We conducted a receiver operating characteristic (ROC) curve analysis to determine the cut-off values for the CONUT score. At each value, an ROC curve was created by plotting the sensitivity and specificity of each result under study. The score closest to the point with both maximum sensitivity and specificity was chosen as the cutoff value, and the largest number of tumors was correctly classified for clinical outcome. There was a minor difference between the optimal cut-off values of RFS and those of OS, and we therefore used the cut-off values of the RFS for the OS in order to maintain consistency and prevent confusion.
The clinicopathological factors examined were as follows: sex, age, cancer location site (right side vs. left side), histology [tub 1 and tub 2 vs. others (por and pap etc.)], preoperative CONUT score, mGPS score, PNI, presence/absence of vascular invasion, presence/absence of lymph invasion pT category (T1, T2 vs. ≥ T3), pN category, tumor size, preoperative CEA level, preoperative CA19-9 level, and early complications.
Ethical approval
The present study was conducted in accord with the Declarations of Helsinki and was approved by the Ethics Committee of the Teikyo University (approval date, 23 August 2016, Registration Number; 16–032).
Abbreviations
- AIC:
-
Akaike's information criterion
- AJCC:
-
American Joint Committee on Cancer
- CA19-9:
-
Carbohydrate antigen 19-9
- CEA:
-
Carcinoembryonic antigen
- CONUT:
-
Controlling Nutritional Status
- CRC:
-
Colorectal cancer
- CRP:
-
C-reactive protein
- mGPS:
-
modified Glasgow Prognostic Score
- OS:
-
Overall survival
- PNI:
-
Prognostic nutritional index
- RFS:
-
Relapse-free survival
References
Favoriti, P. C. G., Greco, M., Pirozzi, F., Pirozzi, R. E. M. & Corcione, F. Worldwide burden of colorectal cancer: a review. Updates Surg.68, 7–11 (2016).
Tokunaga, R. et al. CONUT: a novel independent predictive score for colorectal cancer patients undergoing potentially curative resection. Int. J. Colorectal Dis.32(1), 99–106 (2017).
Daitoku, N. et al. Controlling nutritional status (CONUT) score is a prognostic marker in metastatic colorectal cancer patients receiving first-line chemotherapy. Anticancer Res.38(8), 4883–4888 (2018).
Minami, S., Ogata, Y., Ihara, S., Yamamoto, S. & Komuta, K. Pretreatment Glasgow prognostic score and prognostic nutritional index predict overall survival of patients with advanced small cell lung cancer. Lung Cancer (Auckland, NZ).8, 249–257 (2017).
Miyake, M. et al. Integrative assessment of pretreatment inflammation-, nutrition-, and muscle-based prognostic markers in patients with muscle-invasive bladder cancer undergoing radical cystectomy. Oncology93(4), 259–269 (2017).
Watanabe, J. et al. Prognostic indicators based on inflammatory and nutritional factors after pancreaticoduodenectomy for pancreatic cancer. Surg. Today46(11), 1258–1267 (2016).
Iseki, Y. et al. Impact of the preoperative controlling nutritional status (CONUT) score on the survival after curative surgery for colorectal cancer. PLoS ONE10(7), e0132488 (2015).
Yamamoto, M. et al. Prognostic value of combined tumor marker and controlling nutritional status (CONUT) score in colorectal cancer patients. Yonago Acta Med.62(1), 124–130 (2019).
McMillan, D. C. et al. Measurement of the systemic inflammatory response predicts cancer-specific and non-cancer survival in patients with cancer. Nutr. Cancer.41(1–2), 64–69 (2001).
Hardt, J. et al. Preoperative hypoalbuminemia is an independent risk factor for increased high-grade morbidity after elective rectal cancer resection. Int. J. Colorectal Dis.32(10), 1439–1446 (2017).
Cohen, S., Danzaki, K. & MacIver, N. J. Nutritional effects on T-cell immunometabolism. Eur. J. Immunol.47(2), 225–235 (2017).
Hayama, T. et al. Significance of the 7th postoperative day neutrophil-to-lymphocyte ratio in colorectal cancer. Int. J. Colorectal Dis.35, 119–124 (2019).
Falanga, A., Marchetti, M. & Vignoli, A. Coagulation and cancer: biological and clinical aspects. J. Thromb. Haemost. JTH11(2), 223–233 (2013).
Jin, L. J. et al. Analysis of factors potentially predicting prognosis of colorectal cancer. World J. Gastrointest. Oncol.11(12), 1206–1217 (2019).
Gadgil, M. D., Anderson, C. A., Kandula, N. R. & Kanaya, A. M. Dietary patterns are associated with metabolic risk factors in South Asians living in the United States. J. Nutr.145(6), 1211–1217 (2015).
Ko, K. et al. Influence of nutritional deficiency on prognosis of renal cell carcinoma (RCC). BJU Int.112(6), 775–780 (2013).
Cubiella, J. et al. Prognostic factors in nonresectable pancreatic adenocarcinoma: a rationale to design therapeutic trials. Am. J. Gastroenterol.94(5), 1271–1278 (1999).
Zhou, P., Li, B., Liu, B., Chen, T. & Xiao, J. Prognostic role of serum total cholesterol and high-density lipoprotein cholesterol in cancer survivors: a systematic review and meta-analysis. Clin. Chim. Acta Int. J. Clin. Chem.477, 94–104 (2018).
de Ulibarri Perez, J. I., Fernandez, G., Rodriguez Salvanes, F. & Diaz Lopez, A. M. Nutritional screening; control of clinical undernutrition with analytical parameters. Nutr. Hosp.29(4), 797–811 (2014).
Allott, E. H. et al. Serum cholesterol levels and tumor growth in a PTEN-null transgenic mouse model of prostate cancer. Prostate Cancer Prostatic Dis.21(2), 196–203 (2018).
Nozoe, T. K. M. et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. SurgToday42, 532–535 (2012).
MacDonald, N. Terminology in cancer cachexia: importance and status. Curr. Opin. Clin. Nutr. Metab. Care15(3), 220–225 (2012).
Costa, R. G. F. et al. Cancer cachexia induces morphological and inflammatory changes in the intestinal mucosa. J. Cachexia Sarcopenia Muscle10(5), 1116–1127 (2019).
Krarup, P. M., Nordholm-Carstensen, A., Jorgensen, L. N. & Harling, H. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann. Surg.259(5), 930–938 (2014).
Mynster, T., Christensen, I. J., Moesgaard, F. & Nielsen, H. J. Effects of the combination of blood transfusion and postoperative infectious complications on prognosis after surgery for colorectal cancer. Danish RANX05 Colorectal Cancer Study Group. Br. J. Surg.87(11), 1553–1562 (2000).
The American Joint Committee on Cancer. AJCC Cancer Staging Manual (Springer, New York, 2018).
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int. J. Surg. (Lond., Engl.)12(12), 1495–1499 (2014).
Ignacio de Ulibarri, J. et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp.20(1), 38–45 (2005).
McMillan, D. C., Crozier, J. E., Canna, K., Angerson, W. J. & McArdle, C. S. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int. J. Colorectal Dis.22(8), 881–886 (2007).
Tominaga, T. et al. Prognostic value of the preoperative prognostic nutritional index in oldest-old patients with colorectal cancer. Surg. Today50, 449–459 (2019).
Onodera, T. G. N. & Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi85(9), 1001–1005 (1984).
Ge, X. et al. The role of exclusive enteral nutrition in the preoperative optimization of laparoscopic surgery for patients with Crohn’s disease: a cohort study. Int. J. Surg. (Lond. Engl.)65, 39–44 (2019).
Shimomura, M. et al. Prognostic nutritional index predicts treatment outcomes following palliative surgery for colorectal adenocarcinoma. J. Anus Rectum Colon1(4), 118–124 (2017).
Vrieze, S. I. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol. Methods17(2), 228–243 (2012).
Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control19, 716–723 (1974).
Acknowledgements
21th Fujii Tomoko academic encouragement award
Author information
Authors and Affiliations
Contributions
T.H. made substantial contribution to conception, conducted a literature search and drafted the manuscript. T.H., Y.H. and T.O. made the contribution for acquisition of data. T.H., Y.H., K.N., M.T., Y.O., Y.F., Y.O., S.F., R.S. and M.K. reviewed the manuscript and gave final approval for publication. All authors read and approved the final manuscript
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hayama, T., Ozawa, T., Okada, Y. et al. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients undergoing resection for colorectal cancer. Sci Rep 10, 13239 (2020). https://doi.org/10.1038/s41598-020-70252-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70252-2
This article is cited by
-
Prognostic significance of the Naples prognostic score in colorectal cancer patients undergoing curative resection: a propensity score matching analysis
BMC Gastroenterology (2023)
-
Association between controlling nutritional status score and chronic kidney disease in diabetic patients: a cross-sectional study based on the National Health and Nutrition Examination Survey
International Urology and Nephrology (2023)
-
The preoperative geriatric nutritional risk index (GNRI) is an independent prognostic factor in elderly patients underwent curative resection for colorectal cancer
Scientific Reports (2022)
-
The presurgical controlling nutritional status (CONUT) score is independently associated with severe peristomal skin disorders: a single-center retrospective cohort study
Scientific Reports (2021)
-
Combined prognostic nutritional index and albumin-bilirubin grade to predict the postoperative prognosis of HBV-associated hepatocellular carcinoma patients
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.