Abstract
Fatty liver disease (FLD) increases the risk of diabetes, cardiovascular disease, and steatohepatitis, which leads to fibrosis, cirrhosis, and hepatocellular carcinoma. Thus, the early detection of FLD is necessary. We aimed to find a quantitative and feasible model for discriminating the FLD, based on plasma free amino acid (PFAA) profiles. We constructed models of the relationship between PFAA levels in 2,000 generally healthy Japanese subjects and the diagnosis of FLD by abdominal ultrasound scan by multiple logistic regression analysis with variable selection. The performance of these models for FLD discrimination was validated using an independent data set of 2,160 subjects. The generated PFAA-based model was able to identify FLD patients. The area under the receiver operating characteristic curve for the model was 0.83, which was higher than those of other existing liver function-associated markers ranging from 0.53 to 0.80. The value of the linear discriminant in the model yielded the adjusted odds ratio (with 95% confidence intervals) for a 1 standard deviation increase of 2.63 (2.14–3.25) in the multiple logistic regression analysis with known liver function-associated covariates. Interestingly, the linear discriminant values were significantly associated with the progression of FLD, and patients with nonalcoholic steatohepatitis also exhibited higher values.
Similar content being viewed by others
Introduction
Due to changes in lifestyles, the prevalence of fatty liver disease (FLD) has been consistently increasing worldwide especially in Asian regions1,2,3. Patients with FLD frequently do not exhibit signs or subjective symptoms until their illness becomes advanced, and such patients are at risk of developing steatohepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma2,4,5,6. FLD also increases the risk of developing insulin resistance, diabetes, and cardiovascular diseases7. Thus, the early detection of FLD, which triggers earlier lifestyle modification, is necessary. FLD can be identified and diagnosed by abdominal ultrasound scans. However, the use of ultrasound as a diagnostic method is problematic because the inspection takes time and is unlikely to be conducted during a routine physical examination. Instead, subjects with suspected liver damage are initially screened by biochemical analyses, including the assessment of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) serum level8. Because these enzymes are released from damaged hepatocytes into the blood following hepatocellular injury or death, they are not specific biomarkers of a fatty liver8.
In clinical settings, biomarkers generated from metabolomics are becoming one of the most important diagnostic criteria that can be objectively measured and evaluated as indicators of normal or pathological states, as well as a tool for assessing the outcome of therapeutic interventions. Focused-metabolomics, with well-managed sample collection, and accurate and reproducible measurements appear to be a realistic approach9. One of the traditional examples using a combination of plasma free amino acid (PFAA) profiles for diagnostic markers is Fischer’s ratio, which is the ratio of branched-chain amino acids (BCAAs: valine, leucine, isoleucine) to aromatic amino acids (AAAs: tyrosine, phenylalanine). Fischer’s ratio is used as a diagnostic marker for assessing liver metabolism, hepatic functional reserve, and the severity of liver dysfunction10,11. As liver dysfunction progresses, the plasma levels of BCAAs are decreased and AAAs are increased, respectively, thereby decreasing Fischer’s ratio.
Recent technological advances in focused-metabolomics have uncovered several metabolic signatures associated with FLD, including plasma amino acids12,13. However, to date, few quantitative studies have been performed in a large population, especially with respect to the optimal combination of PFAAs to evaluate the presence of FLD. In this study, we aimed to find a quantitative and feasible model for discriminating the FLD, based on PFAA profiles and confirmed the association with the progression of FLD.
Results
Construction of the robust PFAA- based fatty liver model (FLM)
We constructed a robust PFAA-only-based FLM using the data from 2,000 subjects to identify FLD patients. The performance of this model was confirmed in the independent validation data set of 2,160 subjects. The training data set consisted of 2,000 individuals (1,151 males and 849 females), 465 of whom were diagnosed as having FLD (Table 1). The validation data set consisted of 2,160 individuals (1,320 males and 840 females), 499 of whom were diagnosed as having FLD. Table 2 presents the concentrations of PFAAs. The levels of alanine (Ala), histidine (His), isoleucine (Ile), leucine (Leu), lysine (Lys), methionine (Met), ornithine (Orn), phenylalanine (Phe), proline (Pro), tryptophan (Trp), tyrosine (Tyr), and valine (Val) were significantly higher in patients with FLD, whereas the levels of citrulline (Cit), glycine (Gly), and serine (Ser) were lower in patients with FLD. In the FLM, which was chosen with regard to Akaike’s information criterion (AIC) and the likelihood ratio test, asparagine (Asn), Gly, Ala, Leu, Tyr, and arginine (Arg) were selected as explanatory variables. The amino acids which were elevated, reduced, and sustained in the patients with FLD were selected in this model. The resultant FLM was used for the following validation and characterization.
Discriminant capability of the PFAA- based FLM
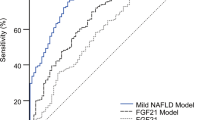
The performance of the linear discriminant in the FLM and liver function-associated markers for discrimination between FLD patients and HCs was evaluated with the use of the receiver-operating-characteristic (ROC) curve and the estimate with 95% confidence interval (CI) of the area under the ROC curve (ROC_AUC). Between FLD patients and HCs, the ROC_AUC of the linear discriminant in the FLM was 0.84 (95% CI: 0.82, 0.86) in the training data set and 0.83 (95% CI: 0.81 to 0.85) in the validation data set (Fig. 1A,B). This ROC_AUC was statistically higher (Delong’s test: p < 0.001) than those of the following liver function-associated markers whose discriminating capabilities were as follows: the ROC_AUCs of gamma-glutamyl transpeptidase (γ-GTP), AST, Fischer’s ratio, alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) were 0.72 (95% CI: 0.69 to 0.74), 0.66 (95% CI: 0.64 to 0.69), 0.60 (95% CI: 0.58 to 0.63), 0.58 (95% CI: 0.56 to 0.61), and 0.53 (95% CI: 0.50 to 0.56), respectively. The ROC_AUC of the linear discriminant in the FLM was also higher (Delong’s test: p < 0.01) than that of ALT whose ROC_AUC was 0.80 (95% CI: 0.78 to 0.82). Table 3 shows sensitivity, specificity, positive predictive value, negative predictive value, and efficiency of the linear discriminant in the FLM.
The discriminant capability of the plasma free amino acid-based model for fatty liver disease and association with metabolic dysfunction. (A) The ROC curves of the fatty liver model (FLM), ALT, γ-GTP, AST, Fischer’s ratio, ALP, and LDH for the discrimination between patients with fatty liver disease (FLD) and healthy subjects. (B) The values of the areas under the ROC curves (ROC_AUC) for discriminating the patients with FLD from healthy subjects. The top and bottom of the boxes indicate the 95% confidence interval of ROC_AUCs. (C) Relationship between the FLM value and the accumulation of risk factors associated with metabolic dysfunction. The FLM value was plotted against the number of risk factors associated with metabolic dysfunction (hypertension, hyperglycemia, hyperlipidemia, and visceral obesity). The box extends from the 25th to 75th percentiles. The line in the middle of the box is plotted at the median. The whiskers are drawn down to the 1st percentile and up to the 99th. Points below and above the whiskers are drawn as individual dots. The significant upward trend was illustrated by the Jonckheere-Terpstra trend test. (D) Box plot of FLM values for healthy controls (HC), patients with FLD, and patients with nonalcoholic steatohepatitis (NASH). ALT: alanine aminotransferase, γ-GTP: gamma-glutamyl transpeptidase, AST: aspartate aminotransferase, ALP: alkaline phosphatase, and LDH: lactate dehydrogenase.
The odds ratios (ORs) for the FLM values for discriminating FLD are presented in Table 4. In the unadjusted models, the ORs associated with a 1 standard deviation (SD) increase in FLM value was 4.97 (95% CI: 4.23 to 5.84). The quintile analysis revealed a trend towards increased OR according to the FLM value (likelihood-ratio test: p < 0.001), and the ORs of top quintile group compared with the bottom quintile group were 48.79 (27.68 to 86.01). Adjusting for the variables affecting the likelihood of FLD (age, sex, AST, ALT, LDH, ALP, and γ-GTP), similar results were obtained, and the ORs associated with a 1 SD increase in FLM value was 4.96 (95% CI: 4.13 to 5.95). The ORs of the top quintile group compared with the bottom quintile group was 44.51 (21.40 to 92.60). These associations remained statistically significant after adjusting for following covariates: age, sex, AST, ALT, LDH, ALP, γ-GTP, weight, BMI, waist circumference, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), total protein (TP), total cholesterol (T-CHO), Fasting plasma glucose (FPG), hemoglobin A1C (HbA1c), homeostasis model assessment of insulin resistance (HOMA-IR), systolic blood pressure (SBP), and diastolic blood pressure (DBP). The ORs associated with 1 SD increase in the FLM value was 2.63 (95% CI: 2.14 to 3.25). The quintile analysis revealed a trend towards increased OR according to the FLM value, and the ORs of the top quintile group compared with the bottom quintile group was 8.16 (95% CI: 3.49 to 19.07). We have also confirmed that similar performance was obtained using the training data set (Supplemental Figure 2). It suggests that the PFAA-based FLM was useful for identifying patients at high risk for FLD, even after adjusting for commonly accepted risk factors.
The association of high FLM value with aggravating conditions of metabolic syndrome
The FLM value was significantly associated with the accumulation of metabolic syndrome-associated risk factors (Fig. 1C). The Jonckheere-Terpstra trend test demonstrated that the FLM value was elevated with the accumulation of risk factors (p < 0.001), suggesting that the FLM could identify subjects with risk factors to metabolic syndrome.
Furthermore, the subjects with nonalcoholic steatohepatitis (NASH) also exhibited higher FLM values compared with HCs (Fig. 1D). The FLM values for NASH patients were as high as those for patients with FLD, both of which were significantly higher than the values in HCs (Kruskal-Wallis test: p < 0.001, Dunn’s multiple comparisons test: p < 0.01 for HCs vs FLD patients; p < 0.05 for HCs vs NASH patients).
Discussion
In this study, we used a focused metabolomic approach with well-managed sample collection and measurements with respect to reproducibility to generate a PFAA-based FLM. The FLM was able to discriminate between FLD patients and HCs even after adjusting for commonly accepted risk factors including age, sex, AST, ALT, LDH, ALP, γ-GTP, weight, BMI, waist circumference, HDL-C, LDL-C, TG, TP, T-CHO, FPG, HbA1c, HOMA-IR, SBP, and DBP (Table 4). The quintile analysis revealed a trend towards increased ORs according to the FLM value, and the OR of the top quintile compared with the bottom quintile was 8.16 (95% CI: 3.49 to 19.07) even after controlling for generally accepted risk factors. In this study, 465 of 2,000 subjects (23.3%) were classified as having FLD in the training data set, and 499 of 2,160 subjects (23.1%) were classified as having FLD in the validation data set (Table 1). Since it is reported that 26.2% of subjects who underwent comprehensive medical check-up were FLD by abdominal ultrasound scan14, the present population could be considered as a general Japanese population.
We recently reported the statistical approach called “AminoIndex technology”9,15,16,17 to compress multidimensional information from the PFAA profile into a single score to maximize the differences between the case and control population. It has been used to discriminate various physiological or disease states, including the progression of liver fibrosis in chronic hepatitis C patients15, visceral obesity and comorbidities18,19,20, and the identification of a risk for cardiovascular disease in diabetic patients21. In order for a biomarker to be successful in clinical settings, it must surpass conventional methods with respect to reliability and discriminative capability and/or should be more informative about disease progression9.
The ROC_AUC of the linear discriminant in the FLM for identifying patients with FLD was higher than that of other liver function-associated markers, demonstrating a competitive edge over existing markers (Fig. 1A,B). If the liver is injured, the liver cells release ALT, γ-GTP, and AST into the blood, thereby raising the plasma levels and signaling the liver damage. Thus, these enzymes are sensitive indicators of liver damage caused by different types of disease but are not specific indicators for liver fat accumulation. Fischer’s ratio, which is the molar ratio of BCAAs to AAAs, is important for assessing the clinical stage of liver disease10,11. Fischer’s ratio decreases as hepatic fibrosis or hepatic dysfunction progresses and thus reflects the degree of hepatic impairment, however, Fischer’s ratio does not decrease as liver fat accumulates. More specifically, the BCAA levels were elevated in subjects with FLD and NASH in this study, which is consistent with previous reports of the elevation of peripheral BCAAs12,13, whereas the BCAA levels decrease as the hepatic impairment progresses in the later stage of liver diseases. Thus the obtained FLM and Fischer’s ratio are completely different. The important next step is to examine the appropriate cutoff point of FLM value and to compare the accuracy of the model with existing markers. Although we calculated sensitivity, specificity, positive and negative predictive values, and efficiency in Table 3, we have to examine the clinical usefulness by comparing to existing markers with appropriate cutoff points in the next study.
The PFAA alterations observed in these FLD patients (Table 2) might have been caused by both metabolic changes due to FLD and dietary habits. However, studies so far have suggested that metabolic shifts, rather than dietary habits, play a more significant role in PFAA alterations. Many reports suggest that PFAA profiles are altered by visceral obesity18,19,20 and insulin resistance19,22,23,24,25, resulting in an elevations in plasma BCAAs, AAAs, and Ala and a decrease in Gly levels. Tai et al.23 previously examined PFAA profiles in association with insulin resistance and dietary habit in 263 non-obese Chinese and Asian-Indian men and demonstrated that although dietary protein intake markedly differed between ethnic groups, it did not affect the PFAA levels. Rather, PFAA levels were strongly associated with HOMA-IR values. Paradoxically, a higher dietary intake of BCAAs has been reported to be related to a lower prevalence of being overweight or obese26, lower insulin resistance27, and decreased risk of diabetes28.
The reason for the increase in blood BCAAs in patients with FLD is thought to be due to the lower uptake of BCAAs into the muscles caused by decreased insulin action and decreased utilization of amino acids in muscles29,30. Moreover, BCAAs are metabolized in visceral adipose tissues as well, and insulin resistance causes a decrease in the expression of BCAA-catabolizing enzymes in adipose tissue29,31,32. Intriguingly, the FLM value was significantly associated with the progression of FLD in this study, and the patients with NASH also exhibited higher FLM values (Fig. 1C,D). Greater elevation in FLM value was associated with higher likelihood for the co-occurrence of metabolic risk factors (visceral obesity, dysglycemia, high blood pressure, and dyslipidemia). Thus, the change in PFAA profiles which is reflected in FLM seems to be metabolic shifts caused by FLD and concomitant insulin resistance. The levels of Gly and Ser, two glucogenic amino acids, were lower in FLD patients. One possible reason for the reduction of Gly and Ser is an increased consumption of these amino acids by enhanced gluconeogenesis33 together with a decreased production by the enhancement of glyceroneogenesis. It has been reported that glucose production from both Gly and Ser in hepatocytes is increased in diabetic individuals, whereas this type of glucose production is low in healthy conditions. Although the levels of several other amino acids, such as Ala, and Cit, changed in FLD patients, the reasons are unclear. Thus, the mechanisms and physiological meaning underlying the PFAA alterations require further investigation.
Although we generated a PFAA-based FLM to identify FLD patients, there are two limitations in this study. The first limitation is lack of alcoholic intake data. The FLD comprises alcoholic and non-alcoholic FLD. Since the pathology between non-alcoholic vs alcoholic FLD are different, PFAA profiles might be different as well. Thus, the future studies to investigate whether the current FLM can evaluate both non-alcoholic and alcoholic FLD are wanted. The second limitation is population bias. Since this FLM was generated in Japanese population which has relatively high FLD ratio14, the performance of the FLM might be different in other populations.
In conclusion, our focused-metabolomic approach generated FLM, which conferred independent and differing contributions to increasing fatty liver risk relative to the currently known risk factors. Further longitudinal studies involving the sequential monitoring to examine the association between the FLM value and liver disease development, such as later NASH stages, fibrosis, cirrhosis, or hepatocellular carcinoma could be of importance for clarifying the physiological meaning of PFAA alterations.
Methods
Ethics
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethical Committees of Mitsui Memorial Hospital and the Ethical Committees of Shinshu University School of Medicine. All subjects gave their informed consent for inclusion before they participated in the study. All the data were analyzed anonymously throughout the study. The study was registered in the University Hospital Medical Information Network Clinical Trials Registry UMIN000015679.
Control subjects and patients with FLD
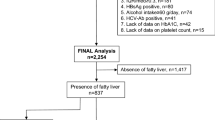
The main inclusion criteria were as follows; generally healthy Japanese subjects with or without FLD who had undergone the Ningen Dock comprehensive medical check-up system34 in 2008 at the Center for Multiphasic Health Testing and Services, Mitsui Memorial Hospital in Tokyo, not taking antidiabetic medications regularly, not having serious health problems, and at least 20 years old (N = 4,160). The patients with hepatitis C or hepatitis B were excluded.
The individuals were divided into two data sets. The first 2,000 chronologically ordered individuals were included in the training data set, and the remaining 2,160 individuals were included in the validation data set. Both training and validation data sets were composed of fatty liver occurrence data (yes = 1, no = 0), PFAA profile data, and demographic and clinical characteristics’ data. The demographic and clinical characteristics of the patients are depicted in Table 1. Among all the data, there were 3 waist circumferences measures missing; therefore, these individuals were not included. Outliers were not excluded from the analyses, since they did not make a significant difference to the results.
NASH patients
NASH patients (N = 10) who had undergone liver biopsy at the Shinshu University School of Medicine who have alcoholic intake data were recruited (Supplementary Table 1). Informed consent was obtained from each patient included in the study. For diagnosing NASH, liver biopsy specimens were obtained from segment 5 or 8 using 14-G needles and immediately fixed in 10% neutral formalin, embedded in paraffin, cut at 4-µm thickness, and stained with the hematoxylin and eosin or using the Azan–Mallory method. The average length of the samples was 15 ± 3 mm, and the average number of portal tracts found in each sample was 11 ± 4.
Histological findings were assessed by an independent experienced pathologist in a blinded fashion and scored according to the staging/grading system proposed by Kleiner et al.35. The nonalcoholic fatty liver disease histological activity score was calculated as the unweighted sum of the scores for steatosis (0–3), lobular inflammation (0–3), and ballooning (0–2). The histological diagnosis of steatohepatitis was made by the presence of macrovesicular steatosis, hepatocyte ballooning, and lobular inflammation.
Analyses of biochemical variables and quantification of PFAAs
Blood samples were taken from the individuals after an overnight fast. FPG, and serum levels of T-CHO, HDL-C, LDL-C, TG, TP, and HbA1c were determined. Serum insulin levels were measured immunologically, and the HOMA-IR was calculated. The SBP and DBP were measured. Biochemical variables related to liver condition were measured, including AST, ALT, γ-GTP, ALP, and LDH. Liver fat content was examined by the ultrasound hepatic/renal ratio. The visceral fat area (VFA) was calculated from computed tomography images, and a 75 g oral glucose tolerance test was performed to obtain their 2-h post-challenge glucose levels for detecting the risk of diabetes in 865 subjects. The measurements of other variables were performed as previously described19,20,36.
For the amino acid analyses, blood samples (5 mL) were collected from forearm veins after overnight fasting into tubes containing disodium ethylenediaminetetraacetate and were immediately placed on ice. The plasma amino acid concentrations were measured by high-performance liquid chromatography–electrospray ionization mass spectrometry followed by precolumn derivatization as previously described37,38,39,40,41. The following 19 amino acids were measured: Ala, Arg, Asn, Cit, glutamine, Gly, His, Ile, Leu, Lys, Met, Orn, Phe, Pro, Ser, threonine, Trp, Tyr, and Val.
Definition of metabolic syndrome related risk factors
According to the available biochemical data and computed tomography (CT) scanning data, the metabolic syndrome risk factors included hypertension, hyperglycemia, hyperlipidemia, and VFA were defined. The following criteria were used: hypertension, SBP equal to or higher than 140 mmHg or DBP equal to or higher than 90 mmHg; hyperglycemia, FPG equal to or higher than 110 mg/dL or blood glucose level equal to or higher than 140 mg/dL after oral glucose tolerance test; hyperlipidemia, LDL-C equal to or higher than 140 mg/dL or TG equal to or higher than 150 mg/dL; and visceral obesity, VFA by CT scan equal to or higher than 100 cm2. For VFA data, the number of individuals for whom the data were available was 865.
Statistical analysis
Description and comparison of demographic and clinical characteristics
Demographic and clinical characteristics are presented as means with SDs, and were compared between the healthy controls (HCs) who had no apparent FLD and FLD patients with the use of Welch’s t-test.
Construction of a robust PFAA-based FLM
To construct a robust PFAA-only-based FLM that discriminated between FLD patients and HCs, a multiple logistic regression model were fitted with leave-one-out cross-validation (LOOCV) to fatty liver occurrence data (response variable) and PFAA profile data (explanatory variables) in the training data set. All the possible combinations of 19 kinds of PFAAs were tried under the constraint that the maximum number of PFAAs was less than seven to avoid a potential over-fitting issue and to attain a parsimonious model. The LOOCV consisted of the following steps: (a) one sample was omitted sequentially one by one from the data set; (b) the logistic regression model was estimated using the data in which one sample was omitted; (c) based on the estimated model, the value of linear discriminant for the omitted sample and AIC were calculated; (d) steps (a) to (c) were repeated until all the linear discriminant values of the data set were obtained. Consequently, the top 100 best-fit models were picked up with regard to AIC.
To obtain the best-fit model that was less affected by age and gender, the model with minimum AIC value was chosen as the final FLM, under the constraint that no statistically significant effects of incorporating age and sex were confirmed with the use of the likelihood-ratio test. Also, each effect of the PFAAs included in the final model was assessed with the use of Wald’s test.
Validation and clinical characterization of FLM
Using the validation data set and NASH patients’ data set, the values of the linear discriminant in the obtained FLM (FLM values) were calculated, and then the FLM was validated as follows: (1) The performance of the linear discriminant in the FLM and liver function-associated markers for discrimination between FLD patients and HCs was evaluated with the use of the ROC curve and the estimate with 95% CI of the ROC_AUC. The ROC_AUC was compared with the use of Delong’s test42. Sensitivity (=num. of true positives/num. of FLD patients), specificity (=num. of true negatives/num. of HCs), positive predictive values (PPV = num. of true positives/num. of positive subjects), negative predictive values (NPV = num. of true negatives/num. of negative subjects), and efficiency (=num. of true positives and true negatives/num. of total subjects) were also estimated with the use of prevalence rate at 23.1% because 499 of 2,160 subjects were classified as having FLD in this validation data set, according to the quintile of the distribution of the FLM values; (2) Relationship between fatty liver occurrence and linear discriminant was assessed with the use of the logistic regression model with/without adjustment of demographic variables and metabolic syndrome related variables, where the FLM values were transformed to z-score and classified into five quintile groups. The ORs are presented with their 95% CIs and p values. Trend towards increased ORs among the five quintile groups was confirmed with the use of likelihood-ratio test; (3) Association of trend towards higher FLM values with the cumulative number of metabolic syndrome-related risk factors (summed up from 0 to 4 in each subject) was assessed with the use of the Jonckheere–Terpstra test; (4) Difference in the distribution of the FLM value among HCs, FLD patients, and NASH patients was assessed with the use of the Kruskal-Wallis test, followed by Dunn’s multiple pairwise comparison test.
Software
MATLAB R2015a (The MathWorks, Natick, MA, USA) was used for all statistical analyses. GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA) was used for the ROC analysis. All probability values are two-sided, and value of p < 0.05 was considered statistically significant.
References
Bellentani, S. & Tiribelli, C. The spectrum of liver disease in the general population: lesson from the Dionysos study. J Hepatol 35, 531–537, doi:S0168827801001519 [pii] (2001).
Fan, J. G. et al. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol 43, 508–514, https://doi.org/10.1016/j.jhep.2005.02.042 (2005).
Kojima, S., Watanabe, N., Numata, M., Ogawa, T. & Matsuzaki, S. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol 38, 954–961, https://doi.org/10.1007/s00535-003-1178-8 (2003).
Dimitroulis, D. et al. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J Gastroenterol 23, 5282–5294, https://doi.org/10.3748/wjg.v23.i29.5282 (2017).
Takeuchi, M., Takino, J. I., Sakasai-Sakai, A., Takata, T. & Tsutsumi, M. Toxic AGE (TAGE) Theory for the Pathophysiology of the Onset/Progression of NAFLD and ALD. Nutrients 9, https://doi.org/10.3390/nu9060634 (2017).
Wiest, R., Albillos, A., Trauner, M., Bajaj, J. & Jalan, R. Targeting the gut-liver axis in liver disease. J Hepatol, https://doi.org/10.1016/j.jhep.2017.05.007 (2017).
Gastaldelli, A. et al. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology 49, 1537–1544, https://doi.org/10.1002/hep.22845 (2009).
Green, R. M. & Flamm, S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology 123, 1367–1384, doi:S001650850200241X [pii] (2002).
Imaizumi, A. et al. In Metabolomics (ed Dr Ute Roessner) 289–314 (InTech, 2012).
Fischer, J. E. et al. The role of plasma amino acids in hepatic encephalopathy. Surgery 78, 276–290, doi:0039-6060(75)90214-7 [pii] (1975).
Soeters, P. B. & Fischer, J. E. Insulin, glucagon, aminoacid imbalance, and hepatic encephalopathy. Lancet 2, 880–882, doi: S0140-6736(76)90541-9 [pii] (1976).
Barr, J. et al. Obesity-dependent metabolic signatures associated with nonalcoholic fatty liver disease progression. J Proteome Res 11, 2521–2532, https://doi.org/10.1021/pr201223p (2012).
Kalhan, S. C. et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 60, 404–413, https://doi.org/10.1016/j.metabol.2010.03.006 (2011).
Okanoue, T., Umemura, A., Yasui, K. & Itoh, Y. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in Japan. J Gastroenterol Hepatol 26(Suppl 1), 153–162, https://doi.org/10.1111/j.1440-1746.2010.06547.x (2011).
Kimura, T., Noguchi, Y., Shikata, N. & Takahashi, M. Plasma amino acid analysis for diagnosis and amino acid-based metabolic networks. Curr Opin Clin Nutr Metab Care 12, 49–53, https://doi.org/10.1097/MCO.0b013e3283169242 (2009).
Miyagi, Y. et al. Plasma free amino acid profiling of five types of cancer patients and its application for early detection. PLoS One 6, e24143, https://doi.org/10.1371/journal.pone.0024143 (2011).
Noguchi, Y. et al. Network analysis of plasma and tissue amino acids and the generation of an amino index for potential diagnostic use. Am J Clin Nutr 83, 513S–519S, doi: 83/2/513S [pii] (2006).
Nagao, K. & Yamakado, M. The role of amino acid profiles in diabetes risk assessment. Curr Opin Clin Nutr Metab Care 19, 328–335, https://doi.org/10.1097/MCO.0000000000000305 (2016).
Yamakado, M. et al. Plasma Free Amino Acid Profiles Predict Four-Year Risk of Developing Diabetes, Metabolic Syndrome, Dyslipidemia, and Hypertension in Japanese Population. Sci Rep 5, 11918, https://doi.org/10.1038/srep11918 (2015).
Yamakado, M. et al. Plasma amino acid profile is associated with visceral fat accumulation in obese Japanese subjects. Clin Obes 2, 29–40, https://doi.org/10.1111/j.1758-8111.2012.00039.x (2012).
Kume, S. et al. Predictive properties of plasma amino acid profile for cardiovascular disease in patients with type 2 diabetes. PLoS One 9, e101219, https://doi.org/10.1371/journal.pone.0101219 (2014).
Nakamura, H. et al. Plasma amino acid profiles are associated with insulin, C-peptide and adiponectin levels in type 2 diabetic patients. Nutr Diabetes 4, e133, https://doi.org/10.1038/nutd.2014.32 (2014).
Tai, E. S. et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 53, 757–767, https://doi.org/10.1007/s00125-009-1637-8 (2010).
Wurtz, P. et al. Metabolic signatures of insulin resistance in 7,098 young adults. Diabetes 61, 1372–1380, https://doi.org/10.2337/db11-1355 (2012).
Wurtz, P. et al. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 36, 648–655, https://doi.org/10.2337/dc12-0895 (2013).
Qin, L. Q. et al. Higher branched-chain amino acid intake is associated with a lower prevalence of being overweight or obese in middle-aged East Asian and Western adults. J Nutr 141, 249–254, https://doi.org/10.3945/jn.110.128520 (2011).
Jennings, A., MacGregor, A., Pallister, T., Spector, T. & Cassidy, A. Associations between branched chain amino acid intake and biomarkers of adiposity and cardiometabolic health independent of genetic factors: A twin study. Int J Cardiol 223, 992–998, https://doi.org/10.1016/j.ijcard.2016.08.307 (2016).
Nagata, C. et al. Branched-chain amino acid intake and the risk of diabetes in a Japanese community: the Takayama study. Am J Epidemiol 178, 1226–1232, https://doi.org/10.1093/aje/kwt112 (2013).
Lynch, C. J. & Adams, S. H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 10, 723–736, https://doi.org/10.1038/nrendo.2014.171 (2014).
Pozefsky, T. et al. Amino acid balance across tissues of the forearm in postabsorptive man. Effects of insulin at two dose levels. J Clin Invest 48, 2273–2282, https://doi.org/10.1172/JCI106193 (1969).
Herman, M. A., She, P., Peroni, O. D., Lynch, C. J. & Kahn, B. B. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J Biol Chem 285, 11348–11356, https://doi.org/10.1074/jbc.M109.075184 (2010).
Lackey, D. E. et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab 304, E1175–1187, https://doi.org/10.1152/ajpendo.00630.2012 (2013).
Hetenyi, G. Jr., Anderson, P. J., Raman, M. & Ferrarotto, C. Gluconeogenesis from glycine and serine in fasted normal and diabetic rats. Biochem J 253, 27–32 (1988).
Ikeda, N. et al. What has made the population of Japan healthy? Lancet 378, 1094–1105, https://doi.org/10.1016/S0140-6736(11)61055-6 (2011).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321, https://doi.org/10.1002/hep.20701 (2005).
Yamamoto, H. et al. Reference intervals for plasma-free amino acid in a Japanese population. Ann Clin Biochem 53, 357–364, https://doi.org/10.1177/0004563215583360 (2016).
Shimbo, K. et al. Automated precolumn derivatization system for analyzing physiological amino acids by liquid chromatography/mass spectrometry. Biomed Chromatogr 24, 683–691, https://doi.org/10.1002/bmc.1346 (2010).
Shimbo, K., Oonuki, T., Yahashi, A., Hirayama, K. & Miyano, H. Precolumn derivatization reagents for high-speed analysis of amines and amino acids in biological fluid using liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 23, 1483–1492, https://doi.org/10.1002/rcm.4026 (2009).
Shimbo, K., Yahashi, A., Hirayama, K., Nakazawa, M. & Miyano, H. Multifunctional and highly sensitive precolumn reagents for amino acids in liquid chromatography/tandem mass spectrometry. Anal Chem 81, 5172–5179, https://doi.org/10.1021/ac900470w (2009).
Takehana, S. et al. The effects of pre-analysis sample handling on human plasma amino acid concentrations. Clin Chim Acta 455, 68–74, https://doi.org/10.1016/j.cca.2016.01.026 (2016).
Yoshida, H. et al. Validation of an analytical method for human plasma free amino acids by high-performance liquid chromatography ionization mass spectrometry using automated precolumn derivatization. J Chromatogr B Analyt Technol Biomed Life Sci 998–999, 88–96, https://doi.org/10.1016/j.jchromb.2015.05.029 (2015).
DeLong, E. R., DeLong, D. M. & Clarke-Pearson, D. L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44, 837–845 (1988).
Acknowledgements
We thank the following people: Dr. M. Takahashi, Dr. Y. Noguchi, Dr. T. Ando, Dr. T. Muramatsu, Dr. M. Mori, and Ms. Y. Kageyama of Ajinomoto Co., Inc., for their useful discussions; Dr. H. Yoshida, Ms. M. Nakamura, Dr. K. Shimbo, and Ms. N. Kageyama of Ajinomoto Co., Inc. for the amino acid analyses; and Ms. N. Takahashi, Ms. M. Takasu, Ms. H. Jinzu, Ms. A. Ikeda, Ms. M. Yamamoto, and Dr. N. Kawai of Ajinomoto Co., Inc. for the data acquisition.
Author information
Authors and Affiliations
Contributions
The authors’ contributions were as follows: M.Y. and K.N. designed research; M.Y., M.K., M.T., A.T., H.Y., and Y.I. conducted research; H.M. provided essential materials and conducted research; T.T., K.N., A.I., T.D., and K.H. analyzed data; M.Y., T.T., and K.N. wrote paper; and K.N. was primarily responsible for final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
M.Y., M.K., M.T., A.T., K.H., and Y.I. received research grants from Ajinomoto Co., Inc. T.T., K.N., A.I., H.M., and H.Y. are employees of Ajinomoto Co., Inc. T.D. received consultancy fees from Ajinomoto Co., Inc. This does not alter the authors’ adherences to all of the journal policies. No other potential conflicts of interest in relation to this article are declared.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamakado, M., Tanaka, T., Nagao, K. et al. Plasma amino acid profile associated with fatty liver disease and co-occurrence of metabolic risk factors. Sci Rep 7, 14485 (2017). https://doi.org/10.1038/s41598-017-14974-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-14974-w
This article is cited by
-
Longitudinal multi-omics analyses of the gut–liver axis reveals metabolic dysregulation in hepatitis C infection and cirrhosis
Nature Microbiology (2022)
-
Associations between plasma sulfur amino acids and specific fat depots in two independent cohorts: CODAM and The Maastricht Study
European Journal of Nutrition (2022)
-
Determination of amino acids in human biological fluids by high-performance liquid chromatography: critical review
Amino Acids (2021)
-
Preoperative AminoIndex Cancer Screening (AICS) abnormalities predict postoperative recurrence in patients undergoing curative resection for non-small cell lung cancer
BMC Cancer (2020)
-
Ursodeoxycholic acid improves liver function via phenylalanine/tyrosine pathway and microbiome remodelling in patients with liver dysfunction
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.