Abstract
The L-type amino acid transporter 1 (LAT1 or SLC7A5) transports large neutral amino acids across the membrane and is crucial for brain drug delivery and tumor growth. LAT1 forms a disulfide-linked heterodimer with CD98 heavy chain (CD98hc, 4F2hc or SLC3A2), but the mechanism of assembly and amino acid transport are poorly understood. Here we report the cryo-EM structure of the human LAT1–CD98hc heterodimer at 3.3-Å resolution. LAT1 features a canonical Leu T-fold and exhibits an unusual loop structure on transmembrane helix 6, creating an extended cavity that might accommodate bulky amino acids and drugs. CD98hc engages with LAT1 through the extracellular, transmembrane and putative cholesterol-mediated interactions. We also show that two anti-CD98 antibodies recognize distinct, multiple epitopes on CD98hc but not its glycans, explaining their robust reactivities. These results reveal the principles of glycoprotein-solute carrier assembly and provide templates for improving preclinical drugs and antibodies targeting LAT1 or CD98hc.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The atomic coordinates of LAT1–CD98hc–MEM-108 Fab and CD98hc-ED–HBJ127 Fab–MEM-108 Fab have been deposited in the Protein Data Bank under accession numbers 6JMQ and 6JMR. The cryo-EM density maps have been deposited in the Electron Microscopy Data Bank under accession numbers EMD-9849 and EMD-9850. The raw micrographs used for this study are available at the Electron Microscopy Public Image Archive under accession codes EMPIAR-10264 and EMPIAR-10265. Source data for Fig. 3d,e and Supplementary Figs. 1d–g and 5a–c are available online. All other data are available from the corresponding author upon request.
References
Kandasamy, P., Gyimesi, G., Kanai, Y. & Hediger, M. A. Amino acid transporters revisited: new views in health and disease. Trends Biochem. Sci. 43, 752–789 (2018).
Kanai, Y. et al. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J. Biol. Chem. 273, 23629–23632 (1998).
Mastroberardino, L. et al. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature 395, 288–291 (1998).
Fotiadis, D., Kanai, Y. & Palacín, M. The SLC3 and SLC7 families of amino acid transporters. Mol. Aspects Med. 34, 139–158 (2013).
Jack, D. L., Paulsen, I. T. & Saier, M. H. The amino acid/polyamine/organocation (APC) superfamily of transporters specific for amino acids, polyamines and organocations. Microbiology 146, 1797–1814 (2000).
Palacín, M. & Kanai, Y. The ancillary proteins of HATs: SLC3 family of amino acid transporters. Pflügers Arch. 447, 490–494 (2004).
Palacin, M., Errasti-Murugarren, E. & Rosell, A. Heteromeric amino acid transporters. In search of the molecular bases of transport cycle mechanisms. Biochem. Soc. Trans. 44, 745–752 (2016).
Yanagida, O. et al. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim. Biophys. Acta Biomembr. 1514, 291–302 (2001).
Ritchie, J. W. A. & Taylor, P. M. Role of the System L permease LAT1 in amino acid and iodothyronine transport in placenta. Biochem. J. 356, 719–725 (2001).
Kageyama, T. et al. The 4F2hc/LAT1 complex transports l-DOPA across the blood–brain barrier. Brain Res. 879, 115–121 (2000).
Uchino, H. et al. Transport of amino acid-related compounds mediated by L-type amino acid transporter 1 (LAT1): insights into the mechanisms of substrate recognition. Mol. Pharmacol. 61, 729–737 (2002).
Nii, T. et al. Molecular events involved in up-regulating human Na+-independent neutral amino acid transporter LAT1 during T-cell activation. Biochem. J. 358, 693–704 (2001).
Boado, R. J., Li, J., Nagaya, M., Zhang, C. & Pardridge, W. M. Selective expression of the large neutral amino acid transporter at the blood–brain barrier. Proc. Natl Acad. Sci. USA 96, 12079–12084 (1999).
Jin, S.-E., Jin, H.-E. & Hong, S.-S. Targeting L-type amino acid transporter 1 for anticancer therapy: clinical impact from diagnostics to therapeutics. Expert Opin. Ther. Targets 19, 1319–1337 (2015).
Häfliger, P. et al. The LAT1 inhibitor JPH203 reduces growth of thyroid carcinoma in a fully immunocompetent mouse model. J. Exp. Clin. Cancer Res. 37, 234 (2018).
Wiriyasermkul, P. et al. Transport of 3-fluoro-l-α-methyl-tyrosine by tumor-upregulated L-type amino acid transporter 1: a cause of the tumor uptake in PET. J. Nucl. Med. 53, 1253–1261 (2012).
Oda, K. et al. L-type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer Sci. 101, 173–179 (2010).
Rosilio, C. et al. L-type amino-acid transporter 1 (LAT1): a therapeutic target supporting growth and survival of T-cell lymphoblastic lymphoma/T-cell acute lymphoblastic leukemia. Leukemia 29, 1253–1266 (2015).
Puris, E., Gynther, M., Huttunen, J., Petsalo, A. & Huttunen, K. M. L-type amino acid transporter 1 utilizing prodrugs: how to achieve effective brain delivery and low systemic exposure of drugs. J. Control. Release 261, 93–104 (2017).
Nicklin, P. et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136, 521–534 (2009).
Fenczik, C. A., Sethi, T., Ramos, J. W., Hughes, P. E. & Ginsberg, M. H. Complementation of dominant suppression implicates CD98 in integrin activation. Nature 390, 36349 (1997).
Cantor, J. et al. CD98hc facilitates B cell proliferation and adaptive humoral immunity. Nat. Immunol. 10, 412–419 (2009).
Jungnickel, K. E., Parker, J. L. & Newstead, S. Structural basis for amino acid transport by the CAT family of SLC7 transporters. Nat. Commun. 9, 550 (2018).
Fang, Y. et al. Structure of a prokaryotic virtual proton pump at 3.2 Å resolution. Nature 460, 1040–1043 (2009).
Gao, X. et al. Mechanism of substrate recognition and transport by an amino acid antiporter. Nature 463, 828–832 (2010).
Kowalczyk, L. et al. Molecular basis of substrate-induced permeation by an amino acid antiporter. Proc. Natl Acad. Sci. USA 108, 3935–3940 (2011).
Ilgü, H. et al. Insights into the molecular basis for substrate binding and specificity of the wild-type L-arginine/agmatine antiporter AdiC. Proc. Natl Acad. Sci. USA 113, 10358–10363 (2016).
Fort, J. et al. The structure of human 4F2hc ectodomain provides a model for homodimerization and electrostatic interaction with plasma membrane. J. Biol. Chem. 282, 31444–31452 (2007).
Calonge, M. et al. Cystinuria caused by mutations in rBAT, a gene involved in the transport of cystine. Nat. Genet. 6, 420–425 (1994).
Feliubadaló, L. et al. Non-type I cystinuria caused by mutations in SLC7A9, encoding a subunit (bo, +AT) of rBAT. Nat. Genet. 23, 52–57 (1999).
Chairoungdua, A. et al. Identification of an amino acid transporter associated with the cystinuria-related type II membrane glycoprotein. J. Biol. Chem. 274, 28845–28848 (1999).
Borsani, G. et al. SLC7A7, encoding a putative permease-related protein, is mutated in patients with lysinuric protein intolerance. Nat. Genet. 21, 297–301 (1999).
Tărlungeanu, D. C. et al. Impaired amino acid transport at the blood brain barrier is a cause of autism spectrum disorder. Cell 167, 1481 (2016).
Sigworth, F. J. Principles of cryo-EM single-particle image processing. Microscopy 65, 57–67 (2016).
Wu, S. et al. Fabs enable single particle cryoEM studies of small proteins. Structure 20, 582–592 (2012).
Yagita, H., Masuko, T. & Hashimoto, Y. Inhibition of tumor cell growth in vitro by murine monoclonal antibodies that recognize a proliferation-associated cell surface antigen system in rats and humans. Cancer Res. 46, 1478–1484 (1986).
Rosell, A. et al. Structural bases for the interaction and stabilization of the human amino acid transporter LAT2 with its ancillary protein 4F2hc. Proc. Natl Acad. Sci. USA 111, 2966–2971 (2014).
Jeckelmann, J.-M. & Fotiadis, D. Volta phase plate cryo-EM structure of the human heterodimeric amino acid transporter 4F2hc-LAT2. Int. J. Mol. Sci. 20, 931 (2019).
Verrey, F. et al. CATs and HATs: the SLC7 family of amino acid transporters. Pflügers Arch. 447, 532–542 (2004).
Schweikhard, E. S. & Ziegler, C. M. Current topics in membranes. Curr. Top. Membr. 70, 1–28 (2012).
Krishnamurthy, H., Piscitelli, C. L. & Gouaux, E. Unlocking the molecular secrets of sodium-coupled transporters. Nature 459, 347–355 (2009).
Ilgü, H. et al. Effects of mutations and ligands on the thermostability of the l-arginine/agmatine antiporter AdiC and deduced insights into ligand-binding of human L-type amino acid transporters. Int. J. Mol. Sci. 19, 918 (2018).
Peura, L. et al. Large amino acid transporter 1 (LAT1) prodrugs of valproic acid: new prodrug design ideas for central nervous system delivery. Mol. Pharmacol. 8, 1857–1866 (2011).
Ylikangas, H. et al. Structure–activity relationship study of compounds binding to large amino acid transporter 1 (LAT1) based on pharmacophore modeling and in situ rat brain perfusion. Eur. J. Pharm. Sci. 48, 523–531 (2013).
Chien, H.-C. et al. Reevaluating the substrate specificity of the L-type amino acid transporter (LAT1). J. Med. Chem. 61, 7358–7373 (2018).
Teixeira, S., Grandi, D. S. & Kühn, L. C. Primary structure of the human 4F2 antigen heavy chain predicts a transmembrane protein with a cytoplasmic NH2 terminus. J. Biol. Chem. 262, 9574–9580 (1987).
Hemler, M. E. & Strominger, J. L. Characterization of antigen recognized by the monoclonal antibody (4F2): different molecular forms on human T and B lymphoblastoid cell lines. J. Immunol. 129, 623–628 (1982).
Dickens, D. et al. Modulation of LAT1 (SLC7A5) transporter activity and stability by membrane cholesterol. Sci. Rep. 7, 43580 (2017).
Franca, R., Veljkovic, E., Walter, S., Wagner, C. A. & Verrey, F. Heterodimeric amino acid transporter glycoprotein domains determining functional subunit association. Biochem. J. 388, 435–443 (2005).
Fenczik, C. A. et al. Distinct domains of CD98hc regulate integrins and amino acid transport. J. Biol. Chem. 276, 8746–8752 (2000).
Wagner, C. A., Lang, F. & Bröer, S. Function and structure of heterodimeric amino acid transporters. Am. J. Physiol. Cell Physiol. 281, 93 (2001).
Bröer, A. et al. Association of 4F2hc with light chains LAT1, LAT2 or y + LAT2 requires different domains. Biochem. J. 355, 725–731 (2001).
Nagamori, S. et al. Novel cystine transporter in renal proximal tubule identified as a missing partner of cystinuria-related plasma membrane protein rBAT/SLC3A1. Proc. Natl Acad. Sci. USA 113, 775–780 (2016).
Hayes, G. M. et al. Antitumor activity of an anti-CD98 antibody. Int. J. Cancer 137, 710–720 (2015).
Itoh, K., Inoue, K., Hayashi, H., Suzuki, T. & Masuko, T. Identification of cell proliferation-associated epitope on CD98 oncoprotein using phage display random peptide library. Cancer Sci. 98, 1696–1700 (2007).
Muramatsu, T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J. Biochem. 159, 481–490 (2016).
Bajaj, J. et al. CD98-mediated adhesive signaling enables the establishment and propagation of acute myelogenous leukemia. Cancer Cell 30, 792–805 (2016).
Singh, N. & Ecker, G. F. Insights into the structure, function, and ligand discovery of the large neutral amino acid transporter 1, LAT1. Int. J. Mol. Sci. 19, 1278 (2018).
Drew, D. & Boudker, O. Shared molecular mechanisms of membrane transporters. Annu. Rev. Biochem. 85, 1–30 (2015).
Yan, R., Zhao, X., Lei, J. & Zhou, Q. Structure of the human LAT1–4F2hc heteromeric amino acid transporter complex. Nature 568, 127–130 (2019).
Goehring, A. et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat. Protoc. 9, 2574–2585 (2014).
Rothbauer, U. et al. A versatile nanotrap for biochemical and functional studies with fluorescent fusion proteins. Mol. Cell. Proteomics 7, 282–289 (2008).
Kirchhofer, A. et al. Modulation of protein properties in living cells using nanobodies. Nat. Struct. Mol. Biol. 17, 133–138 (2009).
Geertsma, E. R., Mahmood, N., Schuurman-Wolters, G. K. & Poolman, B. Membrane reconstitution of ABC transporters and assays of translocator function. Nat. Protoc. 3, 256–266 (2008).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7, e42166 (2018).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Emsley, P. & Crispin, M. Structural analysis of glycoproteins: building N-linked glycans with Coot. Acta Crystallogr. D 74, 256–263 (2018).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Itoh, K. et al. Phage display cloning and characterization of monoclonal antibody genes and recombinant fab fragment against the CD98 oncoprotein. Jpn. J. Cancer Res. 92, 1313–1321 (2001).
Brown, A. et al. Tools for macromolecular model building and refinement into electron cryo-microscopy reconstructions. Acta Crystallogr. D 71, 136–153 (2015).
Wei, L. et al. Specific transport of 3-fluoro-l-α-methyl-tyrosine by LAT1 explains its specificity to malignant tumors in imaging. Cancer Sci. 107, 347–352 (2016).
Mirshahi, T., Logothetis, D. E. Sassaroli, M. in Ion Channel Localization. Methods in Pharmacology and Toxicology (eds Lopatin, A.N. & Nichols, C.G.) 215–231 (Springer, 2001).
Ohgaki, R. et al. Essential roles of L-type amino acid transporter 1 in syncytiotrophoblast development by presenting fusogenic 4F2hc. Mol. Cell. Biol. 37, e00427-16 (2017).
Acknowledgements
We thank R. Danev and M. Kikkawa for setting up the cryo-EM infrastructure; K. Ogomori and M. Miyazaki for technical assistance; G. Kasuya, M. Fukuda and R. Taniguchi for discussions; and W. Kühlbrandt for comments on the manuscript. Y.L. was supported by the Toyobo Biotechnology Foundation Fellowship. This work was supported in part by MEXT/JSPS KAKENHI under grant numbers JP16J07405, to Y.L., and JP16H06294, to O.N.; by AMED under grant numbers JP18am0101082, to M.S., JP18gm0810010, to S.N., JP18cm0106131, to Y.K., and JP18am0101115, to O.N.
Author information
Authors and Affiliations
Contributions
Y.L. and O.N. conceived the study. Y.L. performed expression screening, purified LAT1–CD98hc and Fab, prepared the cryo-EM samples and determined the structure. Y.L., T.K., T.Ni., R.I., T.Y. and M.S. collected the cryo-EM data. P.W. and S.N. performed the transport assays using proteoliposomes. C.J., L.Q., R.O., S.O. and Y.K. performed the transport assays using X. laevis oocytes. T.Na assisted with high-resolution cryo-EM data processing with RELION. K.O. purified the proteins for biochemical assays. H.E. provided LAT1 inhibitors and assisted with data interpretation. Y.L. and O.N. wrote the manuscript with contribution from all authors. O.N. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
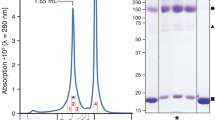
Supplementary Figure 1 Purification and biochemical characterization of LAT1-CD98hc.
a, Size-exclusion chromatography profile of LAT1-CD98hc. The fractions used for structural and functional analyses are indicated by a black bar. b, SDS-PAGE analyses of LAT1-CD98hc with or without 20 mM β-ME. c, Chemical structures of LAT1 inhibitors. d, Uptake of l-[3H]Tyr (50 μM, 10 Ci/mol) by purified LAT1-CD98hc reconstituted in liposomes (cyan) or by control liposomes (white). Proteoliposomes preloaded with 4 mM l-Gln (rectangle) exhibited an initial overshoot of uptake (~1–10 min) as compared to those without preloading (circle), indicating the antiport reaction. The proteoliposomes without preloading also slowly accumulated l-[3H]Tyr, suggesting that LAT1 could adopt facilitative transport mode, in addition to the antiport mode. Values are mean ± SEM. n = 3 technical replicates. e, Substrate specificity of LAT1-CD98hc. Proteoliposomes were incubated with various radiolabeled amino acids and the uptake was measured at 10 min. Control liposomes were used for background subtraction. Although we were not able to measure effective uptake of radiolabeled l-Leu and l-Kyn here due to the high background (Source Data), the competitive inhibition assay confirmed the strong inhibition by l-Leu (f), supporting its transport. Values are mean ± SEM. n = 3 technical replicates. f, Inhibition assay. Proteoliposomes were incubated with l-[3H]Tyr (10 μM) in the presence of 30 mM competitive inhibitors in the external solution. Values are mean ± SEM. n = 3 technical replicates. g, Inhibition assay with pre-incubation of 30 μM SKN-102, SKN-103 and JPH203, or 30 mM BCH. h, Tryptophan-fluorescence size exclusion chromatography of LAT1-CD98hc-Fab complexes. I, Representative 2D class averages of LAT1-CD98hc with or without Fab, recorded on a 200 kV JEM2010F microscope equipped with a CCD camera. Fab molecules are highlighted by yellow asterisks. Note that the box sizes are different between the images with or without Fab.
Supplementary Figure 2 Atomic model of LAT1-CD98hc in the cryo-EM density map.
a, Cryo-EM density maps and atomic models are shown for selected regions. TM1–TM12, IL1 and EL2–EL4 of LAT1 (cyan), TM1’ and the linker of CD98hc (green). The disulfide bond, four N-glycans (orange) and five lipids (yellow). b, Density map of LAT1 in different views. Sterol densities are colored yellow. c, Stereo view of TM1a-TM1b and TM6a-TM6b. The EM map shows no prominent density in the putative substrate-binding site, indicating the apo state. The similar view is shown in Fig. 2c.
Supplementary Figure 3 LAT1-CD98hc interaction.
a, Correlation with previous cross-linking data on LAT2-CD98hc37. Upper, cross-linked pairs are shown on the structure as lines. Dotted lines are for a 60% cross-linked pair. Lower, cross-linked pairs in CD98hc and LAT2 are summarized in a table, along with the corresponding residues in LAT1 and the measured Cα-Cα distances. N/A, not applicable because G220 is disordered, and for visualization I219 is used instead. b, Surface electric potential calculation of LAT1 and CD98hc.
Supplementary Figure 4 Topology of LAT1 and its inward-open state.
a, Side view of LAT1. Helices are colored from blue to red. b, Topology diagram of LAT1. The two large triangles indicate the ‘5+5’ inverted repeats. c, Extracellular and cytoplasmic gates. d, Stereo view of the extracellular gate. e, Stereo view of the cytoplasmic gate. f, Comparison of TM1-TM6-TM10 between LAT1, LAT2, ApcT and AdiC. Unique configuration of the TM6a-TM6b loop underlies the specificity of LAT1. g, Sequence comparison of TM1 and TM6 in LAT1, ApcT and AdiC. The position of Gly255 is indicated by an red arrow. Blue boxes indicate the loop between the two discontinuous helices.
Supplementary Figure 5 Transport assays using X. laevis oocytes.
a, Uptake of l-[14C]Leu by X. laevis oocytes co-expressing CD98hc and LAT1 variants measured at four different substrate concentrations. The data used for Fig. 3d are marked by gray dotted rectangles. The specific radioactivities of l-[14C]Leu are 3.3, 3.3, 2.2 and 0.66 Ci/mol for 50, 100, 300 and 1,000 μM, respectively. Values are mean ± SEM. n = 5–9 technical replicates. b, Uptake of l-[14C]Ala. The specific radioactivities of l-[14C]Ala are 5.6, 5.6, 1.9 and 1.1 Ci/mol for 50, 100, 300 and 1,000 μM Ala uptake, respectively. Values are mean ± SEM. n = 4–9 technical replicates. c, Uptake of various radiolabeled amino acids by X. laevis oocytes co-expressing CD98hc and LAT1 (wild type or G255A) measured at two different substrate concentrations. The data from the 10 µM substrate concentrations are used for Fig. 3e. Values are mean ± SEM. n = 6–9 technical replicates. The specific radioactivities used for 100 µM are 2.2, 4.8, 4.5, 3.3, 20 and 1.1 Ci/mol for l-[14C]Trp, l-[14C]Tyr, l-[14C]Phe, l-[14C]Leu, [3H]Val and l-[14C]Ala, respectively. d, Western blotting of CD98hc and LAT1 variants expressed in X. laevis oocytes. Isolated oocyte membranes were fractionated by SDS-PAGE in the absence of DTT, and blotted with anti-LAT1 or anti-CD98hc antibodies. The results confirmed the heterodimer formation for all variants. The data are representative results from two independent experiments. e, Immunofluorescence of X. laevis oocytes, detected by an anti-LAT1 antibody. The results confirmed the plasma membrane localization for all variants.
Supplementary Figure 6 Full-length CD98hc and lipid interactions.
a, Crystal structure of CD98hc-ED (PDB: 2DH3). It superimposes with the present structure with a root mean square deviation (r.m.s.d.) of 1.31 Å for 414 Cα atoms. b, Cryo-EM structure of CD98hc in the LAT1-CD98hc heterodimer. c, Close-up view of the linker. Atom-atom distances are labeled in gray. Linker residues are labeled in red. d, Close-up view of the sterol binding sites. Densities are contoured at 11 σ.
Supplementary Figure 7 Analysis of SLC3-SLC7 interface.
a, The interaction between CD98hc and LAT1. b, TM1'-TM4 interface. c, Linker-Cβ2-Cβ3-Cβ8-EL2 interface. d, Aα8-EL4a and Aα1-Aα2-EL3 interface. e, Sequence alignment of EL2 and TM4. The conserved 9-amino acid stretch of EL2 is enclosed by a blue box. f, Sequence alignment of EL3. g, Sequence alignment of EL4a-EL4b. h, Sequence alignment of CD98hc and rBAT.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7, Supplementary Notes 1 and 2
Supplementary Video 1
Cryo-EM density map of LAT1 and CD98hc. The 360°-view of the density map and the atomic model are shown for the LAT1 and CD98hc TMD.
Rights and permissions
About this article
Cite this article
Lee, Y., Wiriyasermkul, P., Jin, C. et al. Cryo-EM structure of the human L-type amino acid transporter 1 in complex with glycoprotein CD98hc. Nat Struct Mol Biol 26, 510–517 (2019). https://doi.org/10.1038/s41594-019-0237-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-019-0237-7
This article is cited by
-
Structure and mechanisms of transport of human Asc1/CD98hc amino acid transporter
Nature Communications (2024)
-
Cryo-EM structure of the human Asc-1 transporter complex
Nature Communications (2024)
-
Amino acid metabolism in immune cells: essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy
Journal of Hematology & Oncology (2023)
-
Targeting N-glycosylation of 4F2hc mediated by glycosyltransferase B3GNT3 sensitizes ferroptosis of pancreatic ductal adenocarcinoma
Cell Death & Differentiation (2023)
-
A minority of final stacks yields superior amplitude in single-particle cryo-EM
Nature Communications (2023)