Abstract
Atherosclerosis, the formation of fibrofatty lesions in the artery wall, causes much morbidity and mortality worldwide, including most myocardial infarctions and many strokes, as well as disabling peripheral artery disease. Development of atherosclerotic lesions probably requires low-density lipoprotein, a particle that carries cholesterol through the blood. Other risk factors for atherosclerosis and its thrombotic complications include hypertension, cigarette smoking and diabetes mellitus. Increasing evidence also points to a role of the immune system, as emerging risk factors include inflammation and clonal haematopoiesis. Studies of the cell and molecular biology of atherogenesis have provided considerable insight into the mechanisms that link all these risk factors to atheroma development and the clinical manifestations of this disease. An array of diagnostic techniques, both invasive (such as selective coronary arteriography) and noninvasive (such as blood biomarkers, stress testing, CT and nuclear scanning), permit assessment of cardiovascular disease risk and targeting of therapies. An expanding armamentarium of therapies that can modify risk factors and confer clinical benefit is available; however, we face considerable challenge in providing equitable access to these treatments and in maximizing adherence. Yet, the clinical application of the fruits of research has advanced preventive strategies, enhanced clinical outcomes in affected individuals, and improved their quality of life. Rapidly accelerating knowledge and continued research promise to provide further progress in combating this common chronic disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
World Health Organization. Cardiovascular diseases (CVDs) Fact Sheet. 2017.
Benjamin, E. J. et al. Heart disease and stroke statistics–2017 update: a report from the American Heart Association. Circulation 136, e146–e603 (2017).
Centers for Disease Control and Prevention. Vital signs: prevalence, treatment, and control of high levels of low-density lipoprotein cholesterol–United States, 1999–2002 and 2005–2008. MMWR Morb. Mortal. Wkly Rep. 60, 109–114 (2011).
GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1545–1602 (2016).
Herrington, W., Lacey, B., Sherliker, P., Armitage, J. & Lewington, S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ. Res. 118, 535–546 (2016).
Roth, G. A. et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 132, 1667–1678 (2015).
WHO. Cardiovascular disease: Global Hearts Initiative. (World Health Organization, Geneva, 2018).
Goldstein, J. L. & Brown, M. S. A century of cholesterol and coronaries: from plaques to genes to statins. Cell 161, 161–172 (2015). A telling of the cholesterol tale from two luminaries in the field.
Libby, P. The forgotten majority: unfinished business in cardiovascular risk reduction. J. Am. Coll. Cardiol. 46, 1225–1228 (2005).
Hochholzer, W. & Giugliano, R. P. Lipid lowering goals: back to nature? Ther. Adv. Cardiovasc. Dis. 4, 185–191 (2010).
Giugliano, R. P. et al. Long-term safety and efficacy of achieving very low levels of low-density lipoprotein cholesterol: a prespecified analysis of the IMPROVE-IT trial. JAMA Cardiol. 2, 547–555 (2017).
Hopstock, L. A. et al. Longitudinal and secular trends in total cholesterol levels and impact of lipid-lowering drug use among Norwegian women and men born in 1905–1977 in the population-based Tromso study 1979–2016. BMJ Open. 7, e015001 (2017).
Schreiner, P. J., Jacobs, D. R. Jr., Wong, N. D. & Kiefe, C. I. Twenty-five year secular trends in lipids and modifiable risk factors in a population-based biracial cohort: the Coronary Artery Risk Development in Young Adults (CARDIA) Study, 1985–2011. J. Am. Heart. Assoc. 5, e003384 (2016).
Ference, B. A. et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 38, 2459–2472 (2017).
Nordestgaard, B. G. et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur. Heart J. 34, 3478–3490 (2013).
Cohen, J. C., Boerwinkle, E., Mosley, T. H. Jr. & Hobbs, H. H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 354, 1264–1272 (2006).
Miller, Y. I. et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 108, 235–248 (2011).
Navab, M. et al. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J. Lipid Res. 45, 993–1007 (2004).
Gistera, A. & Hansson, G. K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 13, 368–380 (2017).
Libby, P., Hansson, G. K. & Lichtman, A. H. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity 38, 1092–1104 (2013).
Tardif, J. C. et al. Effects of succinobucol (AGI-1067) after an acute coronary syndrome: a randomised, double-blind, placebo-controlled trial. Lancet 371, 1761–1768 (2008).
Ketelhuth, D. F. J. & Hansson, G. K. Adaptive response of T and B cells in atherosclerosis. Circ. Res. 118, 668–678 (2016). A summary of the roles of adaptive immunity in atherosclerosis.
Boren, J. & Williams, K. J. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: a triumph of simplicity. Curr. Opin. Lipidol. 27, 473–483 (2016).
Llorente-Cortes, V., Martinez-Gonzalez, J. & Badimon, L. LDL receptor-related protein mediates uptake of aggregated LDL in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 20, 1572–1579 (2000).
Musunuru, K. & Kathiresan, S. Surprises from genetic analyses of lipid risk factors for atherosclerosis. Circ. Res. 118, 579–585 (2016). A window into novel aspects of lipids and atherosclerosis emerging from contemporary human genetics.
Libby, P. Triglycerides on the rise: should we swap seats on the seesaw? Eur. Heart J. 36, 774–776 (2015).
Nordestgaard, B. G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ. Res. 118, 547–563 (2016). A rethinking of the contributions of triglyceride-rich lipoproteins to human atherogenesis based on observational epidemiology and human genetics studies.
Burgess, S. et al. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a Mendelian randomization analysis. JAMA Cardiol. 3, 619–627 (2018).
Kranzhofer, R., Browatzki, M., Schmidt, J. & Kubler, W. Angiotensin II activates the proinflammatory transcription factor nuclear factor-kappaB in human monocytes. Biochem. Biophys. Res. Commun. 257, 826–828 (1999).
McMaster, W. G., Kirabo, A., Madhur, M. S. & Harrison, D. G. Inflammation, immunity, and hypertensive end-organ damage. Circ. Res. 116, 1022–1033 (2015).
Rocha, V. Z. & Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 6, 399–409 (2009).
Despres, J. P. Body fat distribution and risk of cardiovascular disease: an update. Circulation 126, 1301–1313 (2012).
Libby, P., Nahrendorf, M. & Swirski, F. K. Leukocytes link local and systemic inflammation in ischemic cardiovascular disease. J. Am. Coll. Cardiol. 67, 1091–1103 (2016).
Libby, P. et al. Inflammation, immunity, and infection in atherothrombosis: JACC Review Topic of the Week. J Am Coll Cardiol. 72, 2071–2081 (2018).
Ridker, P. M. A test in context: high-sensitivity C-reactive protein. J. Am. Coll. Cardiol. 67, 712–723 (2016).
Nus, M. & Mallat, Z. Immune-mediated mechanisms of atherosclerosis and implications for the clinic. Expert Rev. Clin. Immunol. 12, 1217–1237 (2016).
Ignarro, L. J. & Napoli, C. Novel features of nitric oxide, endothelial nitric oxide synthase, and atherosclerosis. Curr. Diab. Rep. 5, 17–23 (2005).
Cybulsky, M. I. & Gimbrone, M. A. Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 251, 788–791 (1991).
Li, H., Cybulsky, M. I., Gimbrone, M. A. Jr. & Libby, P. An atherogenic diet rapidly induces VCAM-1, a cytokine regulatable mononuclear leukocyte adhesion molecule, in rabbit endothelium. Arterioscler. Thromb. 13, 197–204 (1993).
SenBanerjee, S. et al. KLF2 is a novel transcriptional regulator of endothelial proinflammatory activation. J. Exp. Med. 199, 1305–1315 (2004).
Gimbrone, M. A. & García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 118, 620–636 (2016). An up-to-date summary of the roles of endothelial cells in atherosclerosis from a pioneering investigator.
Chatzizisis, Y. S. et al. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol. 49, 2379–2393 (2007).
Bennett, M. R., Sinha, S. & Owens, G. K. Vascular smooth muscle cells in atherosclerosis. Circ. Res. 118, 692–702 (2016).
Robbins, C. S. et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 19, 1166–1172 (2013).
Wanschel, A. et al. Neuroimmune guidance cue semaphorin 3E is expressed in atherosclerotic plaques and regulates macrophage retention. Arterioscler. Thromb. Vasc. Biol. 33, 886–93 (2013).
Swirski, F. K., Nahrendorf, M. & Libby, P. The ins and outs of inflammatory cells in atheromata. Cell. Metab. 15, 135–136 (2012).
Libby, P. & Hansson, G. K. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ. Res. 116, 307–311 (2015).
Gistera, A. et al. Transforming growth factor-β signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci. Transl. Med. 5, 196ra100 (2013).
Grabner, R. et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE-/- mice. J. Exp. Med. 206, 233–248 (2009).
Geng, Y.-J. & Libby, P. Evidence for apoptosis in advanced human atheroma. Co-localization with interleukin-1 beta-converting enzyme. Am. J. Pathol. 147, 251–266 (1995).
Clarke, M. C., Talib, S., Figg, N. L. & Bennett, M. R. Vascular smooth muscle cell apoptosis induces interleukin-1-directed inflammation: effects of hyperlipidemia-mediated inhibition of phagocytosis. Circ. Res. 106, 363–372 (2010).
Tabas, I., Garcia-Cardena, G. & Owens, G. K. Recent insights into the cellular biology of atherosclerosis. J. Cell. Biol. 209, 13–22 (2015).
Yurdagul, A., Doran, A. C., Cai, B., Fredman, G. & Tabas, I. A. Mechanisms and consequences of defective efferocytosis in atherosclerosis. Front. Cardiovasc. Med. 8, 4–86 (2018).
Jaiswal, S. et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 377, 111–121 (2017).
Fuster, J. J. et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355, 842–847 (2017).
Wolach, O. et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci. Transl. Med. 10, eaan8292 (2018).
Libby, P. & Ebert, B. CHIP (clonal hematopoiesis of indeterminate potential): potent and newly recognized contributor to cardiovascular risk. Circulation 138, 666–668 (2018).
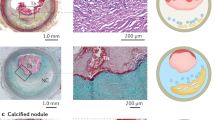
Ruiz, J. L., Hutcheson, J. D. & Aikawa, E. Cardiovascular calcification: current controversies and novel concepts. Cardiovasc. Pathol. 24, 207–212 (2015).
Ruiz, J. L., Weinbaum, S., Aikawa, E. & Hutcheson, J. D. Zooming in on the genesis of atherosclerotic plaque microcalcifications. J. Physiol. 594, 2915–2927 (2016).
Huang, H. et al. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation 103, 1051–1056 (2001).
Irkle, A. et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat. Commun. 6, 7495 (2015).
Galis, Z. et al. Cytokine-stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extracellular matrix digestion. Circ. Res. 75, 181–189 (1994).
Alexander, M. R. et al. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J. Clin. Invest. 122, 70–79 (2012).
Libby, P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 368, 2004–2013 (2013). A consideration of the cellular and molecular mechanisms that underlie the acute coronary syndromes.
Bentzon, J. F., Otsuka, F., Virmani, R. & Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 114, 1852–1866 (2014). An authoritative review of the pathological findings that provide insight into the mechanisms of atherogenesis and its thrombotic complications.
Libby, P. & Pasterkamp, G. Requiem for the ‘vulnerable plaque’. Eur. Heart J. 36, 2984–2987 (2015).
Pasterkamp, G., den Ruijter, H. M. & Libby, P. Temporal shifts in clinical presentation and underlying mechanisms of atherosclerotic disease. Nat. Rev. Cardiol. 14, 21–29 (2017).
Amento, E. P., Ehsani, N., Palmer, H. & Libby, P. Cytokines and growth factors positively and negatively regulate intersitial collagen gene expression in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 11, 1223–1230 (1991).
Galis, Z., Sukhova, G., Lark, M. & Libby, P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Invest. 94, 2493–2503 (1994).
Galis, Z., Sukhova, G., Kranzhöfer, R., Clark, S. & Libby, P. Macrophage foam cells from experimental atheroma constitutively produce matrix-degrading proteinases. Proc. Natl. Acad. Sci. USA 92, 402–406 (1995).
Martinod, K. & Wagner, D. D. Thrombosis: tangled up in NETs. Blood 123, 2768–2776 (2014).
Franck, G. et al. Roles of PAD4 and NETosis in experimental atherosclerosis and arterial injury: implications for superficial erosion. Circ. Res. 123, 33–42 (2018).
Folco, E. J. et al. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1α and cathepsin G. Arterioscler. Thromb. Vasc. Biol. 38, 1901–1912 (2018).
Bevilacqua, M. P., Schleef, R., Gimbrone, M. A. J. & Loskutoff, D. J. Regulation of the fibrinolytic system of cultured human vascular endothelium by IL-1. J. Clin. Invest. 78, 587–591 (1986).
van Lammeren, G. W. et al. Time-dependent changes in atherosclerotic plaque composition in patients undergoing carotid surgery. Circulation 129, 2269–2276 (2014).
Quillard, T., Franck, G., Mawson, T., Folco, E. & Libby, P. Mechanisms of erosion of atherosclerotic plaques. Curr. Opin. Lipidol. 28, 434–441 (2017).
Quillard, T. et al. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur. Heart J. 36, 1394–1404 (2015).
Franck, G. et al. Flow perturbation mediates neutrophil recruitment and potentiates endothelial injury via TLR2 in mice: implications for superficial erosion. Circ. Res. 121, 31–42 (2017).
Fernandez-Friera, L. et al. Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle-aged cohort: the PESA (Progression of Early Subclinical Atherosclerosis) study. Circulation 131, 2104–2113 (2015).
Doukky, R. et al. Promoting appropriate use of cardiac imaging: no longer an academic exercise. Ann. Intern. Med. 166, 438–440 (2017).
Gould, K. L. & Lipscomb, K. Effects of coronary stenoses on coronary flow reserve and resistance. Am. J. Cardiol. 34, 48–55 (1974).
Rumberger, J. A. Coronary artery disease: a continuum, not a threshold. Mayo Clin. Proc. 92, 323–326 (2017).
Topol, E. J. & Nissen, S. E. Our preoccupation with coronary luminology. The dissociation between clincial and angiographic findings in ischemic heart disease. Circulation 92, 2333–2342 (1995).
Tonino, P. A. L. et al. Angiographic versus functional severity of coronary artery stenoses in the fame study. J. Am. Coll. Cardiol. 55, 2816–2821 (2010).
Falk, E., Shah, P. K. & Fuster, V. Coronary plaque disruption. Circulation 92, 657–671 (1995).
Bittencourt, M. S. et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ. Cardiovasc. Imaging. 7, 282–291 (2014).
Maddox, T. M. et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA 312, 1754–1763 (2014).
Wilson, J. M. & Jungner, Y. G. Principles and practice of mass screening for disease [article in Spanish]. Bol. Oficina Sanit. Panam. 65, 281–393 (1968).
Piepoli, M. F. et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 37, 2315–2381 (2016).
US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA 316, 1997–2007 (2016).
Goff, D. C. et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129, S49–S73 (2014).
Stone, N. J. et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129, S1–S45 (2014).
Nasir, K. et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 66, 1657–1668 (2015).
Sabatine, M. S. et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 376, 1713–1722 (2017).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Eikelboom, J. W. et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N. Engl. J. Med. 377, 1319–1330 (2017).
Roth, G. A. et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 70, 1–25 (2017).
Heidenreich, P. A. et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123, 933–944 (2011).
McConnachie, A. et al. Long-term impact on healthcare resource utilization of statin treatment, and its cost effectiveness in the primary prevention of cardiovascular disease: a record linkage study. Eur. Heart J. 35, 290–298 (2014).
Lloyd-Jones, D. M. et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 113, 791–798 (2006).
Falaschetti, E. et al. Adiposity and cardiovascular risk factors in a large contemporary population of pre-pubertal children. Eur. Heart J. 31, 3063–3072 (2010).
Victora, C. G. et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 371, 340–357 (2008).
Juonala, M. et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N. Engl. J. Med. 365, 1876–1885 (2011).
Vedanthan, R. et al. Family-based approaches to cardiovascular health promotion. J. Am. Coll. Cardiol. 67, 1725–1737 (2016).
Ference, B. A. Mendelian randomization studies: using naturally randomized genetic data to fill evidence gaps. Curr. Opin. Lipidol. 26, 566–571 (2015).
Pahkala, K. et al. Ideal cardiovascular health in adolescence: effect of lifestyle intervention and association with vascular intima-media thickness and elasticity (the Special Turku Coronary Risk Factor Intervention Project for Children [STRIP] study). Circulation 127, 2088–2096 (2013).
Koskinen, J. et al. Arterial structure and function after recovery from the metabolic syndrome. The Cardiovascular Risk in Young Finns Study. Circulation 121, 392–400 (2010).
Tonetti, M. S. et al. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 356, 911–920 (2007).
Baena-Diez, J. M. et al. Association between chronic immune-mediated inflammatory diseases and cardiovascular risk. Heart 104, 119–126 (2017).
D’Aiuto, F. et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 6, 954–965 (2018).
D’Aiuto, F. & Deanfield, J. E. Intensive periodontal therapy and type 2 diabetes - Authors’ reply. Lancet Diabetes Endocrinol. 7, 175–176 (2019).
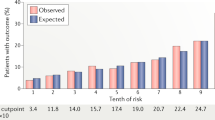
Marma, A. K., Berry, J. D., Ning, H., Persell, S. D. & Lloyd-Jones, D. M. Distribution of 10-year and lifetime predicted risks for cardiovascular disease in US adults: findings from the National Health and Nutrition Examination Survey 2003 to 2006. Circ. Cardiovasc. Qual. Outcomes. 3, 8–14 (2010).
Berry, J. D. et al. Prevalence and progression of subclinical atherosclerosis in younger adults with low short-term but high lifetime estimated risk for cardiovascular disease. The Coronary Artery Risk Development in Young Adults Study and Multi-Ethnic Study of Atherosclerosis. Circulation 119, 382–389 (2009).
Patel, R. S. et al. Online self-assessment of cardiovascular risk using the Joint British Societies (JBS3)-derived heart age tool: a descriptive study. BMJ Open 6, e011511 (2016).
JBS3 Board. Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart 100 (Suppl. 2), ii1–ii67 (2014).
Lopez-Gonzalez, A. A. et al. Effectiveness of the heart age tool for improving modifiable cardiovascular risk factors in a Southern European population: a randomized trial. Eur. J. Prev. Cardiol. 22, 389–396 (2015).
Kivipelto, M. et al. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 5, 735–741 (2006).
Gottesman, R. F. et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 71, 1218–1227 (2014).
Rovio, S. P. et al. Cardiovascular risk factors from childhood and midlife cognitive performance: the Young Finns study. J. Am. Coll. Cardiol. 69, 2279–2289 (2017).
Ngandu, T. et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385, 2255–2263 (2015).
Turakhia, M. P. et al. Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: the Apple Heart Study. Am. Heart J. 207, 66–75 (2019).
Wilkins E. W. L. et al. European Cardiovascular Disease Statistics 2017. (European Heart Network, 2017).
Moran, A. E. et al. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the Global Burden of Disease 2010 study. Circulation 129, 1483–1492 (2014).
Mora, S., Ames, J. M. & Manson, J. E. Low-dose aspirin in the primary prevention of cardiovascular disease: shared decision making in clinical practice. JAMA 316, 709–710 (2016).
Mora, S. & Manson, J. E. Aspirin for primary prevention of atherosclerotic cardiovascular disease: advances in diagnosis and treatment. JAMA Intern. Med. 176, 1195–1204 (2016).
Dugani, S., Ames, J. M., Manson, J. E. & Mora, S. Weighing the anti-ischemic benefits and bleeding risks from aspirin therapy: a rational approach. Curr. Atheroscler. Rep. 20, 15 (2018).
Ridker, P. M. Should aspirin be used for primary prevention in the post-statin era? N. Engl. J. Med. 379, 1572–1574 (2018).
Raber, I. et al. The rise and fall of aspirin in the primary prevention of cardiovascular disease. Lancet 393, 2155–2167 (2019).
Betsholtz, C. et al. cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines. Nature 320, 695–699 (1986).
Chow, C. K. et al. Association of diet, exercise, and smoking modification with risk of early cardiovascular events after acute coronary syndromes. Circulation 121, 750–758 (2010).
Law, M. R., Morris, J. K. & Wald, N. J. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 338, b1665 (2009).
Duckworth, W. et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 360, 129–139 (2009).
Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376, 1670–1681 (2010).
Collins, R. et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 388, 2532–2561 (2016).
Ridker, P. M. What works and in whom? A simple, easily applied, evidence-based approach to guidelines for statin therapy. Circ. Cardiovasc. Qual. Outcomes. 5, 592–593 (2012).
Ridker P. M., Libby P. and Buring J. E. in Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine, 10th Edition (ed. Braunwald, E.) 891–933 (Saunders, 2014).
Grundy, S. M. et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 139, e1082–e1143 (2019).
Di Angelantonio, E. et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302, 1993–2000 (2009).
Saely, C. H., Rein, P. & Drexel, H. Combination lipid therapy in type 2 diabetes. N. Engl. J. Med. 363, 692 (2010). author reply 694–695.
Department of Health and Human Services. Food and Drug Aministration. AbbVie Inc. et al. Withdrawal of approval of indications related to the coadministration with statins in applications for niacin extended-release tablets and fenofibric acid delayed-release capsules. Fed. Regist. 81, 22612–22613 (2016).
Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet 376, 1670–1681 (2010).
Stone, N. J. et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129, S1–S45 (2014).
[No authors listed].The Lipid Research Clinics Coronary Primary Prevention Trial results II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 251, 365–374 (1984).
Hammersley, D. & Signy, M. Ezetimibe: an update on its clinical usefulness in specific patient groups. Ther. Adv. Chronic Dis. 8, 4–11 (2017).
Cannon, C. P. et al. Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 372, 2387–2397 (2015).
Robinson, J. G. et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 372, 1489–1499 (2015).
Sabatine, M. S. et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 372, 1500–1509 (2015).
Nicholls, S. J. et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV Randomized Clinical Trial. JAMA 316, 2373–2384 (2016).
Sabatine, M. S. et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 376, 1713–1722 (2017).
Ridker, P. M. et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N. Engl. J. Med. 376, 1527–1539 (2017).
Ray, K. K. et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N. Engl. J. Med. 376, 1430–1440 (2017).
Giugliano, R. P. et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet 390, 1962–1971 (2017).
Landmesser, U. et al. European Society of Cardiology/European Atherosclerosis Society Task Force consensus statement on proprotein convertase subtilisin/kexin type 9 inhibitors: practical guidance for use in patients at very high cardiovascular risk. Eur. Heart J. 38, 2245–2255 (2017). A masterful review of an important innovation in anti-atherosclerotic therapy with an emphasis on its practical application.
Annemans, L., Packard, C. J., Briggs, A. & Ray, K. K. ‘Highest risk-highest benefit’ strategy: a pragmatic, cost-effective approach to targeting use of PCSK9 inhibitor therapies. Eur. Heart J. 39, 2546–2550 (2018).
Sabatine, M. S. et al. Clinical benefit of evolocumab by severity and extent of coronary artery disease. Circulation 138, 756–766 (2018).
Bhatt, D. L. et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 380, 11–22 (2018).
Antithrombotic Trialists’ Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 324, 71–86 (2002).
Zheng, S. L. & Roddick, A. J. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA 321, 277–287 (2019).
Roffi, M. et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting Without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 37, 267–315 (2016).
Ridker, P. M. et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842 (2017).
Bevilacqua, M. P., Pober, J. S., Majeau, G. R., Cotran, R. S. & Gimbrone, M. A. Jr. Interleukin-1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes and related leukocyte cell lines. J. Clin. Invest. 76, 2003–2011 (1985).
Ridker, P. M. et al. Low-dose methotrexate for the prevention of atherosclerotic events. N. Engl. J. Med. 380, 752–762 (2019).
Nissen S. E. et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N. Engl. J. Med. 375, 2519–2529 (2016).
Ricciotti, E. & FitzGerald, G. A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31, 986–1000 (2011).
Benner JS, G. R., Mogun, H., Neumann, P. J., Weinstein, M. C. & Avorn, J. Long-term persistence in use of statin therapy in elderly patients. JAMA 288, 455–461 (2002).
Kim, M. C. et al. Impact of postdischarge statin withdrawal on long-term outcomes in patients with acute myocardial infarction. Am. J. Cardiol. 115, 1–7 (2015).
Zhang, H., Plutzky, J., Shubina, M. & Turchin, A. Continued statin prescriptions after adverse reactions and patient outcomes: a cohort study. Ann. Intern. Med. 167, 221–227 (2017).
Stroes, E. S. et al. Statin-associated muscle symptoms: impact on statin therapy–European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 36, 1012–1022 (2015).
Newman, C. B. et al. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 39, e38–e81 (2019).
Vaishnava, P. & Lewis, E. F. Assessment of quality of life in severe heart failure. Curr. Heart Fail. Rep. 4, 170–177 (2007).
Mark, D. B. Assessing quality-of-life outcomes in cardiovascular clinical research. Nat. Rev. Cardiol. 13, 286–308 (2016).
Muhammad, I., He, H. G., Kowitlawakul, Y. & Wang, W. Narrative review of health-related quality of life and its predictors among patients with coronary heart disease. Int. J. Nurs. Pract. 22, 4–14 (2016).
Lewis, E. F. et al. Impact of cardiovascular events on change in quality of life and utilities in patients after myocardial infarction: a VALIANT study (valsartan in acute myocardial infarction). JACC Heart Fail. 2, 159–165 (2014).
Thomas, S. B. et al. Racial differences in the association between self-rated health status and objective clinical measures among participants in the BARI 2D trial. Am. J. Public Health 100 (Suppl. 1), S269–S276 (2010).
Sajobi, T. T. et al. Trajectories of health-related quality of life in coronary artery disease. Circ. Cardiovasc. Qual. Outcomes 11, e003661 (2018).
De Smedt, D. et al. Validity and reliability of three commonly used quality of life measures in a large European population of coronary heart disease patients. Int. J. Cardiol. 167, 2294–2299 (2013).
Hlatky, M. A. et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am. J. Cardiol. 64, 651–654 (1989).
Kroenke, K., Spitzer, R. L. & Williams, J. B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16, 606–613 (2001).
Kulik, A. Quality of life after coronary artery bypass graft surgery versus percutaneous coronary intervention: what do the trials tell us? Curr. Opin. Cardiol. 32, 707–714 (2017).
Gomes-Neto, M. et al. High-intensity interval training versus moderate-intensity continuous training on exercise capacity and quality of life in patients with coronary artery disease: a systematic review and meta-analysis. Eur. J. Prev. Cardiol. 24, 1696–1707 (2017).
Allen, J. K. & Dennison, C. R. Randomized trials of nursing interventions for secondary prevention in patients with coronary artery disease and heart failure: systematic review. J. Cardiovasc. Nurs. 25, 207–220 (2010).
De Smedt, D. et al. The association between self-reported lifestyle changes and health-related quality of life in coronary patients: the EUROASPIRE III survey. Eur. J. Prev. Cardiol. 21, 796–805 (2014).
Thomas L. The Lives of a Cell. New York: Penguin Books; 1974: 31–36.
Libby, P., Pasterkamp, G., Crea, F. & Jang, I. K. Reassessing the mechanisms of acute coronary syndromes. Circ. Res. 124, 150–160 (2019).
Acknowledgements
P.L. receives funding support from the National Heart, Lung, and Blood Institute (R01HL080472), the American Heart Association (18CSA34080399), and the RRM Charitable Fund. We thank C. Swallom for her editorial assistance.
Peer review information
Nature Reviews Disease Primers thanks G. Fredman, R. A. Hegele and A. S. Wierzbicki for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Introduction (P.L.); Epidemiology (J.E.B.); Mechanisms/pathophysiology (L.B. and G.K.H.); Diagnosis, screening and prevention (J.D. and M.S.B.); Management (L.T.); Quality of life (E.F.L.); Outlook (P.L.); Overview of Primer (P.L.).
Corresponding author
Ethics declarations
Competing interests
P.L. is an unpaid consultant to, or involved in clinical trials for, Amgen, AstraZeneca, Esperion Therapeutics, Ionis Pharmaceuticals, Kowa Pharmaceuticals, Novartis, Pfizer, Sanofi-Regeneron and XBiotech, Inc. P.L. is a member of scientific advisory boards for Amgen, Corvidia Therapeutics, DalCor Pharmaceuticals, IFM, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune and Novartis. P.L.’s laboratory has received research funding in the last 2 years from Novartis. L.B. has performed lectures and advisory board work in 2017 for Sanofi, Amgen and Astrazeneca. L.B. receives research grant support from AstraZeneca and research funding and grants from Fondo de Investigaciones Sanitarias (FIS), Plan Nacional-Retos MINECO and the EU. G.K.H. is the inventor of patents regarding immune therapy in atherosclerosis. G.K.H. is also the recipient of grants for research on immune mechanisms in atherosclerosis from the Swedish Research Council, the Swedish Heart-Lung Foundation and the EU. J.D. has received CME honoraria and/or consulting fees from Amgen, Boehringer Ingelheim, Merck, Pfizer, Aegerion, Novartis, Sanofi, Takeda, Novo Nordisk and Bayer. J.D. is a member of a Study Steering Committee for Novo Nordisk and has received research grants from the British Heart Foundation, MRC(UK), NIHR, PHE, MSD, Pfizer, Aegerion, Colgate and Roche. M.S.B. has received research support funding from Sanofi and consulting fees from Boston Scientific. L.T. is a member of the scientific advisory boards for Merck, Abbott, Amgen, Sanofi and Daichi Sankyo. L.T. also performed lectures for Abbott, Astra, Actelion, Merck, Servier, Recordati, Mylan, Amgen, Novartis, Sanofi, Pfizer, Bayer, Novo Nordisk and Sanovel. E.F.L. reports institutional research grant and consulting from Novartis, and institutional research grants from Amgen and Sanofi. J.E.B. declares no competing interests.
Rights and permissions
About this article
Cite this article
Libby, P., Buring, J.E., Badimon, L. et al. Atherosclerosis. Nat Rev Dis Primers 5, 56 (2019). https://doi.org/10.1038/s41572-019-0106-z
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-019-0106-z
This article is cited by
-
Emerging applications of single-cell profiling in precision medicine of atherosclerosis
Journal of Translational Medicine (2024)
-
Cardiovascular risk in elderly Egyptians with myelodysplastic syndromes
The Egyptian Journal of Internal Medicine (2024)
-
Investigating the therapeutic effects and mechanisms of Carthamus tinctorius L.-derived nanovesicles in atherosclerosis treatment
Cell Communication and Signaling (2024)
-
The serum soluble ASGR1 concentration is elevated in patients with coronary artery disease and is associated with inflammatory markers
Lipids in Health and Disease (2024)
-
Exploring clinical indicator variations in stroke patients with multiple risk factors: focus on hypertension and inflammatory reactions
European Journal of Medical Research (2024)