Abstract
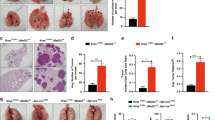
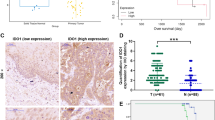
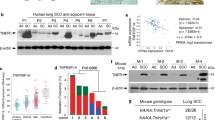
The transcription factor nuclear factor kappa B (NF-κB) has been implicated in having a crucial role in the tumorigenesis of many types of human cancers. Although epidermal growth factor receptor (EGFR) can directly activate NF-κB, the mechanism by which EGFR induces NF-κB activation and the role of NF-κB in EGFR-associated tumor progression is still not fully defined. Herein, we found that mucosa-associated lymphoid tissue 1 (MALT1) is involved in EGFR-induced NF-κB activation in cancer cells, and that MALT1 deficiency impaired EGFR-induced NF-κB activation. MALT1 mainly functions as a scaffold protein by recruiting E3 ligase TRAF6 to IKK complex to activate NF-κB in response to EGF stimulation. Functionally, MALT1 inhibition shows significant defects in EGFR-associated tumor malignancy, including cell migration, metastasis and anchorage-independent growth. To further access a physiological role of MALT1-dependent NF-κB activation in EGFR-driven tumor progression, we generated triple-transgenic mouse model (tetO-EGFRL858R; CCSP-rtTA; Malt1−/−), in which mutant EGFR-driven lung cancer was developed in the absence of MALT1 expression. MALT1-deficient mice show significantly less lung tumor burden when compared with its heterozygous controls, suggesting that MALT1 is required for the progression of EGFR-induced lung cancer. Mechanistically, MALT1 deficiency abolished both NF-κB and STAT3 activation in vivo, which is a result of a defect of interleukin-6 production. In comparison, MALT1 deficiency does not affect tumor progression in a mouse model (LSL-K-rasG12D; CCSP-Cre; Malt1−/−) in which lung cancer is induced by expressing a K-ras mutant. Thus, our study has provided the cellular and genetic evidence that suggests MALT1-dependent NF-κB activation is important in EGFR-associated solid-tumor progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hayden MS, Ghosh S . NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Gene Dev 2012; 26: 203–234.
Karin M . NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol 2009; 1: a000141.
Thome M . Multifunctional roles for MALT1 in T-cell activation. Nat Rev Immunol 2008; 8: 495–500.
Ruland J, Duncan GS, Wakeham A, Mak TW . Differential requirement for Malt1 in T and B cell antigen receptor signaling. Immunity 2003; 19: 749–758.
Ruefli-Brasse AA, French DM, Dixit VM . Regulation of NF-kappaB-dependent lymphocyte activation and development by paracaspase. Science 2003; 302: 1581–1584.
Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L et al. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature 2000; 404: 402–407.
Wang D, You Y, Case SM, McAllister-Lucas LM, Wang L, DiStefano PS et al. A requirement for CARMA1 in TCR-induced NF-kappa B activation. Nat Immunol 2002; 3: 830–835.
Matsumoto R, Wang D, Blonska M, Li H, Kobayashi M, Pappu B et al. Phosphorylation of CARMA1 plays a critical role in T Cell receptor-mediated NF-kappaB activation. Immunity 2005; 23: 575–585.
Sommer K, Guo B, Pomerantz JL, Bandaranayake AD, Moreno-Garcia ME, Ovechkina YL et al. Phosphorylation of the CARMA1 linker controls NF-kappaB activation. Immunity 2005; 23: 561–574.
Blonska M, Lin X . NF-kappaB signaling pathways regulated by CARMA family of scaffold proteins. Cell Res 2011; 21: 55–70.
Akagi T, Motegi M, Tamura A, Suzuki R, Hosokawa Y, Suzuki H et al. A novel gene, MALT1 at 18q21, is involved in t(11;18) (q21;q21) found in low-grade B-cell lymphoma of mucosa-associated lymphoid tissue. Oncogene 1999; 18: 5785–5794.
Dierlamm J, Baens M, Wlodarska I, Stefanova-Ouzounova M, Hernandez JM, Hossfeld DK et al. The apoptosis inhibitor gene API2 and a novel 18q gene, MLT, are recurrently rearranged in the t(11;18)(q21;q21) associated with mucosa-associated lymphoid tissue lymphomas. Blood 1999; 93: 3601–3609.
Morgan JA, Yin Y, Borowsky AD, Kuo F, Nourmand N, Koontz JI et al. Breakpoints of the t(11;18)(q21;q21) in mucosa-associated lymphoid tissue (MALT) lymphoma lie within or near the previously undescribed gene MALT1 in chromosome 18. Cancer Res 1999; 59: 6205–6213.
Ngo VN, Davis RE, Lamy L, Yu X, Zhao H, Lenz G et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature 2006; 441: 106–110.
Coornaert B, Baens M, Heyninck K, Bekaert T, Haegman M, Staal J et al. T cell antigen receptor stimulation induces MALT1 paracaspase-mediated cleavage of the NF-kappaB inhibitor A20. Nat Immunol 2008; 9: 263–271.
Rebeaud F, Hailfinger S, Posevitz-Fejfar A, Tapernoux M, Moser R, Rueda D et al. The proteolytic activity of the paracaspase MALT1 is key in T cell activation. Nat Immunol 2008; 9: 272–281.
Staal J, Driege Y, Bekaert T, Demeyer A, Muyllaert D, Van Damme P et al. T-cell receptor-induced JNK activation requires proteolytic inactivation of CYLD by MALT1. EMBO J 2011; 30: 1742–1752.
Hailfinger S, Nogai H, Pelzer C, Jaworski M, Cabalzar K, Charton JE et al. Malt1-dependent RelB cleavage promotes canonical NF-kappaB activation in lymphocytes and lymphoma cell lines. Proc Natl Acad Sci USA 2011; 108: 14596–14601.
Rosebeck S, Madden L, Jin X, Gu S, Apel IJ, Appert A et al. Cleavage of NIK by the API2-MALT1 fusion oncoprotein leads to noncanonical NF-kappaB activation. Science 2011; 331: 468–472.
Hailfinger S, Lenz G, Ngo V, Posvitz-Fejfar A, Rebeaud F, Guzzardi M et al. Essential role of MALT1 protease activity in activated B cell-like diffuse large B-cell lymphoma. Proc Natl Acad Sci USA 2009; 106: 19946–19951.
Ferch U, Kloo B, Gewies A, Pfander V, Duwel M, Peschel C et al. Inhibition of MALT1 protease activity is selectively toxic for activated B cell-like diffuse large B cell lymphoma cells. J Exp Med 2009; 206: 2313–2320.
Nagel D, Spranger S, Vincendeau M, Grau M, Raffegerst S, Kloo B et al. Pharmacologic inhibition of MALT1 protease by phenothiazines as a therapeutic approach for the treatment of aggressive ABC-DLBCL. Cancer Cell 2012; 22: 825–837.
Fontan L, Yang C, Kabaleeswaran V, Volpon L, Osborne MJ, Beltran E et al. MALT1 small molecule inhibitors specifically suppress ABC-DLBCL in vitro and in vivo. Cancer Cell 2012; 22: 812–824.
Sharma SV, Bell DW, Settleman J, Haber DA . Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007; 7: 169–181.
Yang W, Xia Y, Cao Y, Zheng Y, Bu W, Zhang L et al. EGFR-induced and PKCepsilon monoubiquitylation-dependent NF-kappaB activation upregulates PKM2 expression and promotes tumorigenesis. Mol Cell 2012; 48: 771–784.
Jiang T, Grabiner B, Zhu Y, Jiang C, Li H, You Y et al. CARMA3 is crucial for EGFR-Induced activation of NF-kappaB and tumor progression. Cancer Research 2011; 71: 2183–2192.
Pan D, Lin X . Epithelial growth factor receptor-activated nuclear factor kappaB signaling and its role in epithelial growth factor receptor-associated tumors. Cancer J 2013; 19: 461–467.
Stewart JR, O'Brian CA . Protein kinase C-{alpha} mediates epidermal growth factor receptor transactivation in human prostate cancer cells. Mol Cancer Ther 2005; 4: 726–732.
Fan QW, Cheng C, Knight ZA, Haas-Kogan D, Stokoe D, James CD et al. EGFR signals to mTOR through PKC and independently of Akt in glioma. Sci Signal 2009; 2: ra4.
Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ . The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol Cell 2004; 14: 289–301.
Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest 2007; 117: 3846–3856.
Shambharkar PB, Blonska M, Pappu BP, Li H, You Y, Sakurai H et al. Phosphorylation and ubiquitination of the IkappaB kinase complex by two distinct signaling pathways. EMBO J 2007; 26: 1794–1805.
Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest 2004; 114: 569–581.
Marion S, Mazzolini J, Herit F, Bourdoncle P, Kambou-Pene N, Hailfinger S et al. The NF-kappaB signaling protein Bcl10 regulates actin dynamics by controlling AP1 and OCRL-bearing vesicles. Dev Cell 2012; 23: 954–967.
Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, Ouyang C et al. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature 2009; 462: 104–107.
Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature 2009; 462: 108–112.
Haura EB, Zheng Z, Song L, Cantor A, Bepler G . Activated epidermal growth factor receptor-Stat-3 signaling promotes tumor survival in vivo in non-small cell lung cancer. Clin Cancer Res 2005; 11: 8288–8294.
Songur N, Kuru B, Kalkan F, Ozdilekcan C, Cakmak H, Hizel N . Serum interleukin-6 levels correlate with malnutrition and survival in patients with advanced non-small cell lung cancer. Tumori 2004; 90: 196–200.
Guo Y, Xu F, Lu T, Duan Z, Zhang Z . Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev 2012; 38: 904–910.
Politi K, Zakowski MF, Fan PD, Schonfeld EA, Pao W, Varmus HE . Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Gene Dev 2006; 20: 1496–1510.
Fisher GH, Wellen SL, Klimstra D, Lenczowski JM, Tichelaar JW, Lizak MJ et al. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Gen Dev 2001; 15: 3249–3262.
Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Gene Dev 2001; 15: 3243–3248.
Li H, Cho SN, Evans CM, Dickey BF, Jeong JW, DeMayo FJ . Cre-mediated recombination in mouse Clara cells. Genesis 2008; 46: 300–307.
Acknowledgements
We thank Dr Vishva Dixit (Genentech Corporation) for providing Malt1-deficient mice and Dr Francesco J DeMayo (Baylor College of Medicine) for providing CCSP-Cre mice and Dr Jeffrey Whitsett (Cincinnati Children’s Hospital Medical Center) for providing CCSP-tTA mice. This work is partially supported by grants, RP120316 from Cancer Prevention Research Institute of Texas (CPRIT) to XL, GM079451 and GM065899 from National Institutes of Health (NIH) to XL and R01 CA164346 (NCI/NIH), Developmental Research Awards in Leukemia SPORE CA100632 to MJY, and Center for Inflammation and Cancer, Center for Genetics and Genomics, IRG, Sister Institution Network fund of UT MD Anderson Cancer Center, and Cancer Prevention Research Institute of Texas to MJY.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Pan, D., Jiang, C., Ma, Z. et al. MALT1 is required for EGFR-induced NF-κB activation and contributes to EGFR-driven lung cancer progression. Oncogene 35, 919–928 (2016). https://doi.org/10.1038/onc.2015.146
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.146
This article is cited by
-
In-depth quantitative proteomics analysis revealed C1GALT1 depletion in ECC-1 cells mimics an aggressive endometrial cancer phenotype observed in cancer patients with low C1GALT1 expression
Cellular Oncology (2023)
-
Notch1-ADAM8 positive feed-back loop regulates the degradation of chondrogenic extracellular matrix and osteoarthritis progression
Cell Communication and Signaling (2019)
-
TBKBP1 and TBK1 form a growth factor signalling axis mediating immunosuppression and tumourigenesis
Nature Cell Biology (2019)
-
CARD–BCL-10–MALT1 signalling in protective and pathological immunity
Nature Reviews Immunology (2019)
-
Hectd3 promotes pathogenic Th17 lineage through Stat3 activation and Malt1 signaling in neuroinflammation
Nature Communications (2019)