Abstract
Anti-neutrophil cytoplasmic antibodies (ANCAs) are valuable laboratory markers used for the diagnosis of well-defined types of small-vessel vasculitis, including granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). According to the 1999 international consensus on ANCA testing, indirect immunofluorescence (IIF) should be used to screen for ANCAs, and samples containing ANCAs should then be tested by immunoassays for proteinase 3 (PR3)-ANCAs and myeloperoxidase (MPO)-ANCAs. The distinction between PR3-ANCAs and MPO-ANCAs has important clinical and pathogenic implications. As dependable immunoassays for PR3-ANCAs and MPO-ANCAs have become broadly available, there is increasing international agreement that high-quality immunoassays are the preferred screening method for the diagnosis of ANCA-associated vasculitis. The present Consensus Statement proposes that high-quality immunoassays can be used as the primary screening method for patients suspected of having the ANCA-associated vaculitides GPA and MPA without the categorical need for IIF, and presents and discusses evidence to support this recommendation.
Similar content being viewed by others
Main
Anti-neutrophil cytoplasmic antibodies (ANCAs), such as those directed towards proteinase 3 (PR3) and myeloperoxidase (MPO), are associated with a distinct form of small-vessel vasculitis, known as ANCA-associated vasculitis (AAV), a term that encompases granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). Screening for the presence of ANCAs is a commonly used diagnostic test for AAV. According to an international consensus statement issued in 1999 (Ref. 1), indirect immunofluorescence (IIF) should be used as the initial screening method to detect the presence of ANCAs. Samples that test positive by IIF should then be tested by immunoassays to detect ANCAs specific for PR3 and MPO. Although this testing algorithm is still widely applied, the position of IIF is being questioned.
Over the past 15 years, the performance of enzyme-linked immunosorbent assays (ELISAs) have improved and novel, sensitive and automated technologies, such as fluoroenzyme immunoassays, chemiluminescence assays and multiplexed flow immunoassays, have been introduced. Additionally, assay setup (antigen presentation) has advanced with the development of second generation (capture-based) and third generation (anchor-based) assays. In general, currently available assays for PR3-ANCAs and MPO-ANCAs are highly sensitive and specific for diagnosing GPA and MPA (reviewed elsewhere2).
The availability of reliable antigen-specific immunoassays has raised doubts as to whether the two-stage diagnostic strategy currently recommended for ANCA detection is the best approach2,3. The use of antigen-specific assays as the initial and/or only step has been suggested as an alternative approach to screening by IIF (Refs 4,5). In a 2016 large multicentre study by the European Vasculitis Study Group (EUVAS), the diagnostic performance of antigen-specific immunoassays was confirmed to equal or even to exceed the diagnostic performance of IIF (Ref. 6).
Given the improvements in antigen-specific immunoassays, the international consensus on the testing of ANCAs in small-vessel vasculitis seems in need of updating7,8,9,10. In this manuscript, a revised 2017 international consensus is proposed by a group of international experts (from North and Central America, Australia, Europe and Asia) in the ANCA field. This Consensus Statement highlights the value of ANCA testing as a tool for diagnosis (but not follow-up) of GPA and MPA and gives a historical perspective of ANCAs in small-vessel vasculitis. This Consensus Statement does not, however, present evidence-based guidelines or a meta-analysis.
Methods
This Consensus Statement was prepared by a group of experts from four European laboratories (X.B., J.D., N.R., J.W.C.T. and E.C.) in person and by correspondence. The draft was circulated to each contributor and modified, and the resulting document was distributed by e-mail to 16 experts from four continents, selected based on their expertise and knowledge in clinical and/or laboratory aspects of AAV. This revised document resulted in a second round of discussions and revisions. The final document was returned to all contributors for ratification.
The Consensus Statement is based on the results of a multicentre European Vasculitis Study Group (EUVAS) evaluation of the value of IIF versus antigen-specific immunoassays for ANCA detection6,11,12. This study, which showed a large variability between different IIF methods and a good diagnostic performance of PR3-ANCA and MPO-ANCA immunoassays6, indicated that the 1999 international consensus on ANCA testing for AAV needed revision. When the consensus was put together, the topics that were discussed encompassed IIF versus immunoassays for ANCA detection in GPA and MPA, diagnostic strategies, clinical indications for ANCA testing, value-added reporting of ANCA test results and ANCAs in conditions other than GPA and MPA.
Historical perspective
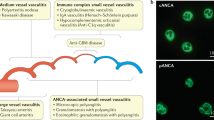
First discoveries in ANCA detection. The history of ANCAs in AAV is depicted in Fig. 1 and has been previously described elsewhere13,14. Although ANCAs were initially discovered in 1959 in patients with chronic inflammatory disorders15, the association between vasculitis (in particular glomerulonephritis) and autoantibodies reacting with cytoplasmic components of neutrophils only became apparent in 1982 (Ref. 16). In 1985, van der Woude et al. detected such anti-cytoplasmic antibodies by IIF in a mixed Dutch–Danish cohort of patients with GPA17, noting that these antibodies produced a cytoplasmic staining pattern (C-ANCA). Following description of C-ANCA, autoantibodies that produce a perinuclear staining pattern (P-ANCA) by IIF were also reported in patients with systemic arteritis and glomerulonephritis18,19; the relevant autoantigens for C-ANCA and P-ANCA were identified as PR3 and MPO, respectively18,20,21,22. ANCAs have subsequently been associated with other small-vessel vasculitides, including MPA, eosinophilic granulomatosis with polyangiitis (EGPA) and idiopathic necrotizing crescentic glomerulonephritis2,3,13,14.
In the past 25 years, substantial progress has been made in the development of assays for detecting anti-neutrophil cytoplasmic antibodies (ANCAs). Achievements have been made in antigen characterization (indicated in green), in the standardization of ANCA assays (indicated in blue), in incorporation of ANCAs in nomenclature and classification proposals (indicated in pink) and in ANCA technology (indicated in grey). Consensus statements on ANCA testing are indicated in orange. In this timeline, the dates for the distinct assays formats concern the publications of commercially available immunoassays. CHCC, Chapel Hill Consensus Conference; C-ANCA, cytoplasmic ANCA staining pattern; ELISA, enzyme-linked immunosorbent assay; GPA, granulomatosis with polyangiitis; IIF, indirect immunofluorescence; MPO, myeloperoxidase; PR3, proteinase 3; P-ANCA, perinuclear ANCA staining pattern.
At first, 'in-house' ELISAs were used for the detection of MPO-ANCAs and PR3-ANCAs. However, following a recognized need for standardization, international efforts were undertaken to develop and standardize solid-phase ANCA assays23,24 (discussed below). In 1998, 15 clinical centres evaluated such standardized assays for the detection of ANCA in patients with idiopathic systemic vasculitis25 (Table 1), concluding that the diagnostic value of ANCA detection by IIF could be greatly enhanced by combining this test with an antigen-specific ELISA. In this study, Hagen et al. showed that ANCA detection by IIF was sensitive for GPA, MPA and renal-limited vasculitis (sensitivities of 81–85%) but had a low specificity (76%). Combining IIF with an ELISA (PR3-ANCA and MPO-ANCA) increased the specificity to 98% and decreased the sensitivities to 67–82%25. The results of this multicentre study were the basis of the 1999 international consensus statement on the testing and reporting of ANCAs1. This 1999 consensus statement states that ANCAs are best demonstrated by using a combination of IIF and immunoassay (PR3-ANCA or MPO-ANCA) and that IIF must be performed on all serum samples of patients suspected of having AAV1. Serum samples containing ANCAs by IIF should then be tested for PR3-ANCAs and MPO-ANCAs1. The testing algorithm proposed by this consensus was validated in 2002 in a meta-analysis study, leading to the conclusion that combining results obtained by IIF and ELISA (combining either C-ANCA and PR3-ANCA or P-ANCA and MPO-ANCA findings) optimizes the diagnostic performance of ANCA testing for AAV26.
Incorporation of ANCA tests in clinical decisions. In the early 1990s, classification criteria and nomenclature for the small-vessel vasculitides were assigned by the American College of Rheumatology27,28 and the Chapel Hill Consensus Conference (CHCC), respectively29. These criteria were based on clinical manifestations and hallmark pathological features of tissue biopsy samples, but did not incorporate ANCA testing. Small-vessel vasculitides were originally considered to be only ANCA-associated, but subsequent animal model studies showed that ANCAs also have pathogenic potential30,31, which was clearly demonstrated for MPO-ANCAs in 2002 (Ref. 32), and later indirectly for PR3-ANCAs33. The fact that different approaches were needed to demonstrate the pathogenic potential of MPO-ANCAs and PR3-ANCAs in these studies increased the awareness that instead of distinguishing between patients with GPA, MPA and EGPA, differentiating between patients with MPO-ANCAs or PR3-ANCAs might be more clinically relevant34,35,36,37. This notion was underscored in 2012 by the finding that these autoantibodies can be used to differentiate between genetically distinct subsets of patients with AAV38. The combined potential pathogenic role of these autoantibodies32,33 and the good test performances of the ANCA-assays26, formed the basis for incorporating ANCAs into nomenclature criteria; in the 2012 CHCC update on the nomenclature of the vasculitides, AAV was included as a category of vasculitis39. Importantly, CHCC is a nomenclature system, not a classification system or a diagnostic system, and at present there are no validated diagnostic criteria for AAV. ANCA detection was included as part of a consensus methodology developed in 2007 for the classification of AAV and polyarteritis nodosa in epidemiological studies40, and EULAR have pointed to considering ANCA in diagnostic and classification criteria for systemic vasculitis41.
Novel technical developments in ANCA detection. Since the description of the first commercial ANCA ELISA in 1990 (Ref. 42), an increasing number of commercial ANCA assays have become available. ELISAs have evolved in the way in which antigens are coupled to the carrier: from direct antigen binding (first generation ELISA) to capture-based antigen binding (second generation ELISA) and anchor-based antigen binding (third generation ELISA)2,3,43,44,45,46,47,48. Similarly, IIF has also undergone technical innovations: neutrophil substrates have been combined with antigen-specific biochip and microbead technology49,50, and automatic pattern recognition devices have become available to support the evaluation of IIF (Refs 51,52). However, innovations in ANCA detection have not only been limited to IIF and ELISAs; alternative solid-phase assays are also now being marketed, including dot and line immunoassays53, fluorescent-enzyme immunoassays (FEIA)54,55, addressable-laser-bead immunoassays (ALBIA)56,57,58,59 and chemiluminescent immunoassays (CLIA)60,61. Many of these assays are reliable methods for ANCA detection, and such advances have challenged the role of IIF in ANCA testing and in the testing algorithm recommended by the 1999 international consensus6.
Harmonization and standardization of ANCA testing. A standard procedure for ANCA IIF was released in 1988 (Ref. 62), prescribing the use of a mixture of neutrophils and other white blood cells smeared on glass slides and fixed with ethanol to differentiate between C-ANCA and P-ANCA, and to determine the ANCA titre of a sample. The result is considered 'not determinable' if the serum contains antinuclear antibodies (ANAs), as detected on human epithelial type 2 (HEp-2) cells, at a similar titre to that determined for P-ANCA. At present, most clinical laboratories use commercial cell substrates for ANCA detection by IIF and, in many cases, neutrophils are used on their own rather than in a mixture with other cells.
Efforts in harmonizing ANCA detection began in 1993 with an international study on the standardization of ANCA assays23. In this multicentre study, the value of IIF and solid-phase techniques (ELISAs) for ANCA detection was evaluated23. The IIF test results across different centres were comparable for sera containing high ANCA titres (even when different methods were used), whereas the results indicated that the ELISAs for PR3-ANCAs were not well-standardized, except when purified PR3 was used as the antigen preparation23. For the MPO ELISAs, various antigen preparations revealed only minor discrepancies in results, with the researchers concluding that all of these MPO preparations could be used in ELISAs for the detection of MPO-ANCAs23.
An addendum to the 1999 international consensus was released in 2003 recommending the use of internal and external quality control procedures in ANCA testing63. In 2007, the first human reference sera for MPO-ANCAs and PR3-ANCAs became available via the Centers for Disease Control and Prevention (CDC). These samples were prepared under the auspices of the International Union of Immunological Societies (IUIS). Each reference preparation was obtained by plasmapheresis from a single donor and was confirmed to be monospecific for either MPO or PR3. To our knowledge, at least four companies currently calibrate their ANCA assays against the IUIS-CDC reference sera: the second generation ANCA ELISAs of Wieslab (Euro-Diagnostica), a third generation ANCA FEIA (Thermo-Fisher), a cytobead IIF assay (Medipan) and a CLIA (Inova). In 2016, the Institute for Reference Materials and Measurements (IRMM) released certified reference material for MPO-ANCAs (ERM-DA476/IFCC)64. This reference material is based on a plasmapheresis sample from a single patient with vasculitis. A similar approach for developing certified reference material for PR3-ANCAs is currently in progress64. Although the use of a certified reference material will reduce variability between ANCA results obtained with different assays, it should be noted that autoantibodies are not a uniform analyte. This caveat holds true for patient sera, as well as for the reference antibody preparations. Antibodies differ in terms of their IgG subclass composition as well as the avidity, glycosylation and epitope specificity of the antibodies65,66,67,68,69,70,71,72,73,74. In particular, epitope specificity might affect standardization of different assay formats, as the accessibility of epitopes might differ between different assay formats. Therefore, the feasibility of using reference antibody preparations for standardization of autoantibody assays (that is, MPO-ANCA and PR3-ANCA assays) remains to be established12.
Rationale for a new consensus
In a 2016 multicentre EUVAS evaluation, the performance of manual and automated IIF was compared with the performance of various antigen-specific immunoassays for ANCA detection6,11. Four European centres contributed samples and clinical data from newly diagnosed patients with GPA (n = 186) and MPA (n = 65) and relevant disease controls (n = 924)6,11. Because ANCA levels might change during treatment, only newly diagnosed patients were included. Eight different antigen-specific immunoassays (from seven manufacturers, encompassing different technological platforms) and four different IIF assays (including two automated assays) were evaluated6,11. As illustrated in Fig. 2, the results of the study revealed a large amount of variability between IIF methods. Moreover, the pattern assignment between IIF methods also varied11. By contrast, immunoassays for PR3-ANCAs and MPO-ANCAs had a high diagnostic performance6. This study, which was performed on diagnostic samples obtained from patients who had not received any immunosuppressive treatment, did not reveal consistent differences between different assay generations and formats. Hence, in contrast to expectations, the improvements in test characteristics were independent of the assay principle. Notably, some patients tested negatively by both IIF and immunoassay, or by either immunoassay or IIF. Depending on the assay, 11−17% of patients with AAV were negative by IIF and 9−16% by immunoassay6. Hence, antigen-specific immunoassays might detect antibodies that are missed by IIF and vice versa6.
This graph depicts the receiver operating characteristics (ROC) curves for different methods of anti-neutrophil cytoplasmic antibody (ANCA) detection by indirect immunofluorescence (IIF) and by myeloperoxidase (MPO)-specific and proteinase 3 (PR3)-specific immunoassays. The figure demonstrates the substantial variation between the IIF methods and the good performance of antigen-specific immunoassays. IIF was performed with ethanol-fixed neutrophils using either the manual Copenhagen approach (blue line) or automated Aklides platform (orange line). IIF was also performed with ethanol-fixed neutrophils in combination with additional tests on formalin-fixed neutrophils and HEp-2 cells using either the manual Bad Bramstedt approach (red line) or automated EuroPattern platform (purple line). Immunoassays were performed using a third generation PR3-ANCA and first generation MPO-ANCA enzyme-linked immunosorbent as says (ELISAs) from Euroimmun (green line). This figure was adapted with permission obtained from Csernok, E., et al. Evaluation of automated multi-parametric indirect immunofluorescence assays to detect anti-neutrophil cytoplasmic antibodies (ANCA) in granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). Autoimmun. Rev. 15, 736–741 (2016).
When compared with the assays used by Hagen et al.25, the antigen-specific immunoassays used in the EUVAS study performed much better, with a higher specificity, demonstrating the marked improvements that have been made to these assays (Table 1). Of note, however, the composition of the control groups differed between the two studies. For example, Hagen et al.25 included patients with inflammatory bowel disease (IBD), whereas Damoiseaux et al. did not6, using instead relevant disease controls for AAV (that is, patients for whom the clinician considered the possibility of AAV and requested ANCA testing, but for whom AAV was eventually excluded), as well as cohorts of patients with a systemic rheumatic disease.
Given the large variability between IIF methods and poor performance of some IIF methods (manual as well as automated), in addition to the good performance of the immunoassays evaluated, the authors of the EUVAS study concluded that screening with IIF and follow-up testing with antigen-specific immunoassay was not necessary for maximal diagnostic accuracy6. These results indicated that the 1999 international consensus on ANCA testing for AAV needed revision7,8,9,10.
New recommendations
In this Consensus Statement, we recommend the use of high-quality immunoassays as the preferred first screening method for GPA and MPA, and put forward a new testing algorithm (recommendations 1–6). These recommendations are visually represented in Fig. 3 and displayed in Box 1.
a | In the 1999 consensus document, the recommended approach for anti-neutrophil cytoplasmic antibody (ANCA) detection was to screen for ANCA by indirect immunofluorescence (IIF) and to test for proteinase 3 (PR3)-ANCAs and myeloperoxidase (MPO)-ANCAs in IIF-positive samples; the ideal approach was to perform IIF and immunoassay on all samples. b | In the 2017 consensus, the use of high-quality immunoassays is recommended as the preferred first screening method for ANCA detection in patients suspected of having the ANCA-associated vaculitides granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). ANCA detection for non-ANCA-associated vasculitis conditions is not included in this consensus. *A second PR3-MPO-ANCA or IIF can be considered for negative results in patients with a high clinical suspicion (to increase sensitivity) or in case of low antibody levels (to increase specificity). Take antibody level into account.
To determine if ANCA testing is advisable, adherence to a strict gating strategy, based on clinical manifestations defined in the 1999 consensus1 (Box 2), is recommended. This strategy strongly reduces the number of ANCA test requests and improves the diagnostic performance of ANCA testing, with fewer false positive results (recommendation 1)75,76,77.
Based on the results of this consensus initiative, there is substantial international agreement that high-quality antigen-specific immunoassays are the preferred screening methodology for the diagnosis of AAV (recommendation 2). IIF is no longer deemed suitable as the first screening test, and adds little additional benefit to antigen-specific assays in the diagnosis of AAV when the pre-test probability for the disease is high6.
Single immunoassays never have a sensitivity and specificity of 100%. In patients where there is a high degree of clinical suspicion and negative ANCA test results, testing by another method can be useful to increase sensitivity (recommendation 3). False-positive results do occur with immunoassays, mainly in samples with a low degree of positivity78. Therefore, performing a second assay or IIF can marginally increase the specificity in cases of low-positive test results10. When new assays are introduced (including assays not included in the EUVAS study6), the diagnostic performance of such assays should be checked based on samples from patients with GPA or MPA and relevant disease controls.
A diagnosis of AAV cannot be excluded for ANCA-negative patients (recommendation 4) and biopsies of the affected organs should be performed in seronegative patients6. Although ANCAs are helpful in the diagnosis of AAV, the diagnosis of AAV should be based on clinicopathological features (recommendation 5).
Interpretation of test results can be improved by the application of appropriately designed reference ranges (and test result intervals) for antibody levels (recommendation 6). The concept of test result interval-specific likelihood ratios is explained in the next section.
The specific role of IIF testing in ANCA testing algorithms should be determined individually by diagnostic laboratories on the basis of the specific clinical need and circumstances of the laboratory. If a laboratory prefers to use IIF as a screening assay in locally determined best-testing algorithms, then the laboratory needs to ensure that the IIF operates at a high level of sensitivity, as the performance of IIF varies greatly between laboratories.
Improving clinical interpretations
As immunoassays are expected to be increasingly used to screen for AAV, retrieving the maximum amount of clinically useful information from PR3-ANCA and MPO-ANCA test results is important. Traditionally, a single cut-off value is employed to predict clinically-relevant reactivity. However, a lot of information is lost when only binary results (positive or negative) are considered, whereas the likelihood for AAV increases with increasing levels of PR3-ANCAs and MPO-ANCAs79.
The likelihood ratio helps to describe the clinical value of a test result. This ratio can be defined for different test result intervals of an assay and is independent of the disease prevalence and pre-test probability. A likelihood ratio of 1 indicates no difference in pre-test to post-test probability, whereas likelihood ratios of >10 or <0.1 indicate large, often clinically important differences in pre-test to post-test probability79,80,81.
A detailed analysis of the large dataset from the multinational EUVAS study6 exemplified and confirmed that the likelihood ratio for AAV increases with increasing levels of PR3-ANCAs and MPO-ANCAs for all immunoassays included in the study78. For example, the likelihood ratio for AAV was calculated to be 0.1, 1.2, 10.2, 64.6, and ∞ for test result intervals of 0–12 CU, 12–24 CU, 24–78 CU, 78–1,050 CU, and 1,050–3,500 CU, respectively, when using the PR3-ANCA and MPO-ANCA QuantaFlash CLIA (Inova)78.
Knowledge of test result-specific likelihood ratios can help clinicians and laboratory professionals to better interpret results. Having the likelihood ratios enables the calculation of the post-test probability for a disease when the pre-test probability is known using the formula: post-test odds = pre-test odds × likelihood ratio78. Figure 4 illustrates the post-test probability for AAV as a function of the pre-test probability (using the formula mentioned above) for different test result intervals. Such graphical representation is a better way to help the interpretation of a test result than describing the sensitivity and specificity of an assay82 and enables the post-test probability to be estimated from the assay test result without the need for a calculation. However, an estimate of the pre-test probability is first required; the pre-test probabilities for AAV associated with particular clinical presentations can be obtained from the literature26,83,84 and have been previously summarized78. For instance, the pre-test probability for AAV in adult patients presenting with haematuria, proteinuria and creatinine levels of 1.5–3 mg/dL is 7%84. If ANCA testing reveals a test result with a likelihood ratio of 60 (for example, a test result between 78–1,050 CU by QuantaFlash), then the post-test probability for AAV will be 82%. By contrast, the post-test probability of a test result with a likelihood ratio of 10.2 or 0.1 will be, respectively, 47% and 0.7%. Such knowledge might add value to a specific test result and help in the clinical interpretation of the result.
The figure exemplifies how a graph can be used in practice to help in the interpretation of test results. The graph shows the post-test probability for anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) as a function of the pre-test probability for test results with a likelihood ratio of 0.1, 1, 10, 60 and ∞. In a previous study78, ANCA test result intervals that have a similar spread of likelihood ratios were delimited for several commercial assays. The equations to calculate post-test probability based on pre-test probability and likelihood ratio are: post-test odds = pre-test odds x likelihood ratio; odds = probability/(1−probability); probability = odds/(1+odds). The graph shows that for a patient with a pre-test probability of AAV of 7%, the post-test probability will be 0.7%, 7%, 43%, 82% and 100% for a test result with a likelihood ratio of 0.1, 1, 10, 60 and ∞, respectively.
Contraindications and considerations
The consensus recommendations proposed in this manuscript are for detecting ANCAs in AAV, in particular GPA and MPA. However, ANCAs can also be found in several other conditions. In this section, we give an overview of these conditions to further help with clinical interpretation of ANCA test results.
ANCAs in other small-vessel vasculitides. ANCAs are also found in 30–38% of patients with EGPA85, a disease characterized by asthma, eosinophilia and granulomatous inflammation, and in 20–35% of patients with anti-glomerular basement membrane (anti-GBM) disease86. The majority of these ANCA-positive patients have MPO-ANCAs87. As the phenotypes of patients with EGPA are heterogeneous, EGPA was not included in this Consensus Statement.
ANCAs in gastrointestinal disorders. In addition to vasculitis, ANCAs are found in patients with gastrointestinal disorders such as IBD88, primary sclerosing cholangitis89 and inflammatory liver diseases (such as autoimmune hepatitis, primary biliary cirrhosis and chronic viral hepatitis)90. These diseases are associated with a slightly aberrant P-ANCA pattern that is often referred to as atypical P-ANCA or X-ANCA88.
In gastrointestinal disorders, P-ANCA is mainly observed in patients with ulcerative colitis (50–67%), but is also seen in patients with Crohn's disease (6–15%), and to a lesser extent in disease controls (<11%)88. Combining P-ANCA with anti-Saccharomyces cerevisiae antibody (ASCA) measurements might improve the clinical utility of this marker. ASCAs are found in 40–60% of patients with Crohn's disease, 4–14% of patients with ulcerative colitis and <5% of controls88. The combination of an ASCA-positive and P-ANCA-negative test result is associated with Crohn's disease, whereas the combination of an ASCA-negative and P-ANCA-positive test result is associated with ulcerative colitis. However, the clinical usefulness of ANCAs in IBD has been questioned. Given the limited sensitivity of ANCA detection in ulcerative colitis, a European evidence-based consensus on the diagnosis and management of ulcerative colitis concluded that routine use of ANCA detection for diagnosis and therapeutic decisions was not clinically justified91.
In the past few years, studies have also reported that sensitive immunoassays can detect PR3-ANCAs in patients with ulcerative colitis50,92,93 and primary sclerosing cholangitis94.
Our consensus recommendations are applicable to AAV, but not to gastrointestinal disorders. As ANCAs can be found in gastrointestinal disorders, we advise differentiating between requesting ANCA tests in the context of AAV and in the context of gastrointestinal disorders (Fig. 3). The coexistence of AAV with IBD has been described in some patients, but is rare95,96. In this context, IBD usually precedes AAV by several years96.
ANCAs in systemic inflammatory and malignant diseases. ANCAs have also been reported in systemic diseases such as rheumatoid arthritis and systemic lupus erythematosus (reviewed elsewhere97). A rare association of AAV with malignant haemopathy (mainly non-Hodgkin lymphoma and myelodysplasia) has additionally been described98.
ANCAs and infection. Evidence suggests that infections have a central role in the formation of ANCAs and that chronic infections mimic AAV99; infective endocarditis can mimic ANCA-associated glomerulonephritis and patients with infective endocarditis can develop ANCAs. Langlois et al.100 reported ANCAs by IIF in 12 out of 50 patients (24%) with infective endocarditis; four patients had PR3-ANCAs, one patient had MPO-ANCAs and two patients had both PR3-ANCAs and MPO-ANCAs. Mahr et al.101 reported ANCA by IIF in 20 out of 109 patients (18%) with infective endocarditis (14 patients were positive for C-ANCA and six were positive for P-ANCA); of these, three patients had C-ANCA and PR3-ANCAs, two patients had C-ANCA and MPO-ANCAs, one patient had P-ANCA and MPO-ANCAs, one patient had just PR3-ANCAs and one patient had just MPO-ANCAs. Misdiagnosis of sub-acute bacterial endocarditis as AAV and initiation of inappropriate immunosuppressive therapy can have devastating consequences102. Thus, infections such as infective endocarditis, hepatitis C infection and tuberculosis should be excluded before establishing a diagnosis of AAV and starting immunosuppressive therapy. The EUVAS multicentre study was performed in Europe, where the prevalence of infections such as malaria, leprosy and tuberculosis is low compared with regions such as India or Mexico, where positive ANCA results have been reported in patients with such infections103,104. Controversy still exists over ANCA positivity in patients with tuberculosis103,104,105.
Drug-induced AAV. Levamisole-adulterated cocaine and drugs such as hydralazine, propylthiouracil and minocycline can cause secondary forms of AAV (reviewed elsewhere106,107). Vasculitis, MPO-ANCAs, PR3-ANCAs, human neutrophil elastase (HNE)-ANCAs and ANAs can all be found in patients with levamisole-adulterated cocaine-induced AAV107.
In a series of 30 patients with AAV associated with cocaine use, all patients had MPO-ANCA and 50% had PR3-ANCAs108; double positivity for MPO-ANCAs and PR3-ANCAs is a characteristic of this disease108. In patients with hydralazine-induced AAV, MPO-ANCAs can be found together with HNE-ANCAs, lactoferrin-ANCAs and ANAs107. In patients with propylthiouracil-mediated AAV, high titres of MPO-ANCAs are usually found107. Of note, a substantial fraction (32–41%) of propylthiouracil-treated patients develop ANCAs (PR3-ANCAs and HNE-ANCAs) without symptoms106,109. In minocycline-induced AAV, P-ANCA is frequently found (∼80% of individuals) with antibody reactivity against either MPO, HNE, bactericidal permeability increasing protein (BPI), lactoferrin or cathepsin G110,111,112,113. Patients are also frequently positive for ANAs.
Taken together, most patients with drug-induced AAV have MPO-ANCA, which can be found in combination with antibodies to other neutrophil cytoplasmic proteins and ANAs.
Conclusion
In the past 25 years, PR3-ANCA and MPO-ANCA assays have evolved from home-made assays (using crude extracts) that have low levels of specificity, to commercially available assays with improved sensitivity and specificity that can be run on automated platforms. A 2016 multicentre EUVAS evaluation demonstrated the good diagnostic performance of current antigen-specific immunoassays for ANCA detection in patients with GPA and MPA, and the high variability in performance of IIF. Our recommendation is that high-quality immunoassays for PR3-ANCAs and MPO-ANCAs are the preferred methods for the diagnostic evaluation of patients with AAV, without the categorical need for IIF. This consensus recommendation applies to ANCA testing for the diagnosis of vasculitis, in particular GPA and MPA, but does not apply to ANCA testing as an adjunct for the diagnosis of IBD, autoimmune hepatitis or drug-induced autoimmunity. In certain settings, infections should be ruled out and a detailed history of medications and illicit drug use should be retrieved. Clinical interpretation of ANCA test results can be improved by taking into account antibody levels and applying test result interval specific likelihood ratios. These recommendations aim to help in the diagnosis of GPA and MPA, and in the standardization of ANCA detection and interpretation. The performance of this consensus recommendation should be evaluated prospectively.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Savige, J. et al. International consensus statement on testing and reporting of antineutrophil cytoplasmic antibodies (ANCA). Am. J. Clin. Pathol. 111, 507–513 (1999).
Csernok, E. & Moosig, F. Current and emerging techniques for ANCA detection in vasculitis. Nat. Rev. Rheumatol. 10, 494–501 (2014).
Cohen Tervaert, J. W. & Damoiseaux, J. Antineutrophil cytoplasmic autoantibodies: how are they detected and what is their use for diagnosis, classification and follow-up? Clin. Rev. Allergy Immunol. 43, 211–219 (2012).
Russell, K. A., Wiegert, E., Schroeder, D. R., Homburger, H. A. & Specks, U. Detection of anti-neutrophil cytoplasmic antibodies under actual clinical testing conditions. Clin. Immunol. 103, 196–203 (2002).
Vermeersch, P. et al. Determination of anti-neutrophil cytoplasmic antibodies in small vessel vasculitis: comparative analysis of different strategies. Clin. Chim. Acta 397, 77–81 (2008).
Damoiseaux, J. et al. Detection of antineutrophil cytoplasmic antibodies (ANCAs): a multicentre European Vasculitis Study Group (EUVAS) evaluation of the value of indirect immunofluorescence (IIF) versus antigen-specific immunoassays. Ann. Rheum. Dis. 76, 647–653 (2017).
Collison, J. Vasculitis syndromes: new ANCA assays put through their paces. Nat. Rev. Rheumatol. 12, 560 (2016).
Damoiseaux, J. et al. Antineutrophil cytoplasmic antibodies: appropriate use and interpretation. Ann. Rheum. Dis. 76, e24 (2017).
Novikov, P., Smitienko, I., Bulanov, N., Zykova, A. & Moiseev, S. Testing for antineutrophil cytoplasmic antibodies (ANCAs) in patients with systemic vasculitides and other diseases. Ann. Rheum. Dis. 76, e23 (2017).
Damoiseaux, J. Csernok, E., Rasmussen, N., Cohen Tervaert, J. W. & Bossuyt, X. Antineutrophil cytoplasmic antibodies: reporting and diagnostic strategies. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2017-211171 (2017).
Csernok, E. et al. Evaluation of automated multi-parametric indirect immunofluorescence assays to detect anti-neutrophil cytoplasmic antibodies (ANCA) in granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). Autoimmun. Rev. 15, 736–741 (2016).
Rasmussen, N. et al. Individual values of antineutrophil cytoplasmic antibodies do not correspond between antigen-specific assays. Clin. Chem. Lab. Med. http://dx.doi.org/10.1515/cclm-2017-0362 (2017)
Cohen Tervaert, J. W. & Damoiseaux, J. Fifty years of antineutrophil cytoplasmic antibodies (ANCA) testing: do we need to revise the international consensus statement on testing and reporting on ANCA. APMIS Suppl. 117 (Suppl. 127), 55–59 (2009).
Rasmussen, N., Wiik, A. & Jayne, D. R. A historical essay on detection of anti-neutrophil cytoplasmic antibodies. Nephrol. Dial. Transplant. 30 (Suppl. 1), i8–i13 (2015).
Calabresi, P., Edwards, E. A. & Schilling, R. F. Fluorescent antiglobulin studies in leukopenic and related disorders. J. Clin. Invest. 38, 2091–2100 (1959).
Davies, D. J., Moran, J. E., Niall, J. F. & Ryan, G. B. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? Br. Med. J. (Clin. Res. Ed.) 285, 606 (1982).
van der Woude, F. J. et al. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet 1, 425–429 (1985).
Falk, R. J. & Jennette, J. C. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N. Engl. J. Med. 318, 1651–1657 (1988).
Cohen Tervaert, J. et al. Wegener's Granulomatosis and anticytoplasmic antibodies: the Groningen experience. APMIS 97 (Suppl. 6), 36 (1989).
Goldschmeding, R. et al. Wegener's granulomatosis autoantibodies identify a novel diisopropylfluorophosphate-binding protein in the lysosomes of normal human neutrophils. J. Clin. Invest. 84, 1577–1587 (1989).
Niles, J. L., McCluskey, R. T., Ahmad, M. F. & Arnaout, M. A. Wegener's granulomatosis autoantigen is a novel neutrophil serine proteinase. Blood 74, 1888–1893 (1989).
Jenne, D. E., Tschopp, J., Ludemann, J., Utecht, B. & Gross, W. L. Wegener's autoantigen decoded. Nature 346, 520 (1990).
Hagen, E. C. et al. The value of indirect immunofluorescence and solid phase techniques for ANCA detection. A report on the first phase of an international cooperative study on the standardization of ANCA assays. J. Immunol. Methods 159, 1–16 (1993).
Hagen, E. C. et al. Development and standardization of solid phase assays for the detection of anti-neutrophil cytoplasmic antibodies (ANCA). A report on the second phase of an international cooperative study on the standardization of ANCA assays. J. Immunol. Methods 196, 1–15 (1996).
Hagen, E. C. et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR project for ANCA assay standardization. Kidney Int. 53, 743–753 (1998).
Choi, H. K., Liu, S., Merkel, P. A., Colditz, G. A. & Niles, J. L. Diagnostic performance of antineutrophil cytoplasmic antibody tests for idiopathic vasculitides: metaanalysis with a focus on antimyeloperoxidase antibodies. J. Rheumatol. 28, 1584–1590 (2001).
Leavitt, R. Y. et al. The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum. 33, 1101–1107 (1990).
Fries, J. F. et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Summ. Arthritis Rheum. 33, 1135–1136 (1990).
Jennette, J. C. et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 37, 187–192 (1994).
Heeringa, P. & Cohen Tervaert, J. W. Pathophysiology of ANCA-associated vasculitides: are ANCA really pathogenic? Kidney Int. 65, 1564–1567 (2004).
Van Timmeren, M. M. & Heeringa, P. Pathogenesis of ANCA-associated vasculitis: recent insights from animal models. Curr. Opin. Rheumatol. 24, 8–14 (2012).
Xiao, H. et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J. Clin. Invest. 110, 955–963 (2002).
Little, M. A. et al. Anti-proteinase 3 anti-neutrophil cytoplasm autoantibodies recapitulate systemic vasculitis in mice with a humanized immune system. PLoS ONE. 7, e28626 (2012).
Franssen, C. F. et al. Antiproteinase 3- and antimyeloperoxidase-associated vasculitis. Kidney Int. 57, 2195–2206 (2000).
Schönermarck, U., Lamprecht, P., Csernok, E. & Gross, W. L. Prevalence and spectrum of rheumatic diseases associated with proteinase 3-antineutrophil cytoplasmic antibodies (ANCA) and myeloperoxidase-ANCA. Rheumatology (Oxford) 40, 178–184 (2001).
Hilhorst, M., van Paassen, P. & Cohen Tervaert, J. W. & Limburg Renal Registry Proteinase 3-ANCA vasculitis versus myeloperoxidase-ANCA vasculitis. J. Am. Soc. Nephrol. 26, 2314–2327 (2015).
Cornec, D., Cornec-Le Gall, E., Fervenza, F. C. & Specks, U. ANCA-associated vasculitis — clinical utility of using ANCA specificity to classify patients. Nat. Rev. Rheumatol. 12, 570–579 (2016).
Lyons, P. A. et al. Genetically distinct subsets within ANCA-associated vasculitis. N. Engl. J. Med. 367, 214–223 (2012).
Jennette, J. C. et al. 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 65, 1–11 (2013).
Watts, R. et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann. Rheum. Dis. 66, 222–227 (2007).
Basu, N. et al. EULAR points to consider in the development of classification and diagnostic criteria in systemic vasculitis. Ann. Rheum. Dis. 69, 1744–1750 (2010).
Rasmussen, N., Sjölin, C., Isaksson, B., Bygren, P. & Wieslander, J. An ELISA for the detection of anti-neutrophil cytoplasm antibodies (ANCA). J. Immunol. Methods 127, 139–145 (1990).
Tervaert, J. W. et al. Autoantibodies against myeloid lysosomal enzymes in crescentic glomerulonephritis. Kidney Int. 37, 799–806 (1990).
Westman, K. W. et al. Clinical evaluation of a capture ELISA for detection of proteinase-3 antineutrophil cytoplasmic antibody. Kidney Int. 53, 1230–1236 (1998).
Lee, A. S. et al. A novel capture-ELISA for detection of anti-neutrophil cytoplasmic antibodies (ANCA) based on c-myc peptide recognition in carboxy-terminally tagged recombinant neutrophil serine proteases. J. Immunol. Methods 307, 62–72 (2005).
Hellmich, B., Csernok, E., Fredenhagen, G. & Gross, W. L. A novel high sensitivity ELISA for detection of antineutrophil cytoplasm antibodies against proteinase-3. Clin. Exp. Rheumatol. 25 (Suppl. 44), S1–S5 (2007).
Damoiseaux, J. et al. A novel enzyme-linked immunosorbent assay using a mixture of human native and recombinant proteinase-3 significantly improves the diagnostic potential for antineutrophil cytoplasmic antibody-associated vasculitis. Ann. Rheum. Dis. 68, 228–233 (2009).
Roggenbuck, D. et al. High-sensitivity detection of autoantibodies against proteinase-3 by a novel third-generation enzyme-linked immunosorbent assay. Ann. NY Acad. Sci. 1173, 41–46 (2009).
Damoiseaux, J. et al. EUROPLUS ANCA BIOCHIP mosaic: PR3 and MPO antigen microdots improve the laboratory diagnostics of ANCA-associated vasculitis. J. Immunol. Methods 348, 67–73 (2009).
Sowa, M. et al. Simultaneous automated screening and confirmatory testing for vasculitis-specific ANCA. PLoS ONE. 9, e107743 (2014).
Damoiseaux, J. et al. Automatic reading of ANCA-slides: evaluation of the AKLIDES system. Clin. Dev. Immunol. 2012, 762874 (2012).
Knütter, I. et al. Automated interpretation of ANCA patterns — a new approach in the serology of ANCA-associated vasculitis. Arthritis Res. Ther. 14, R271 (2012).
Ermens, A. A. et al. Evaluation of a simple dot-blot method for the detection of anti-neutrophil cytoplasmic antibodies directed against proteinase 3 and myeloperoxidase. Clin. Chem. 46, 1717–1719 (2000).
Damoiseaux, J. G. et al. Evaluation of a new fluorescent-enzyme immuno-assay for diagnosis and follow-up of ANCA-associated vasculitis. J. Clin. Immunol. 25, 202–208 (2005).
Sinico, R. A., Radice, A., Corace, C., Di Toma, L. & Sabadini, E. Value of a new automated fluorescence immunoassay (EliA) for PR3 and MPO-ANCA in monitoring disease activity in ANCA-associated systemic vasculitis. Ann. NY Acad. Sci. 1050, 185–192 (2005).
Damoiseaux, J. et al. Evaluation of the FIDIS vasculitis multiplex immunoassay for diagnosis and follow-up of ANCA-associated vasculitis and Goodpasture's disease. Ann. NY Acad. Sci. 1109, 454–463 (2007).
Trevisin, M., Pollock, W., Dimech, W. & Savige, J. Evaluation of a multiplex flow cytometric immunoassay to detect PR3- and MPO-ANCA in active and treated vasculitis, and in inflammatory bowel disease (IBD). J. Immunol. Methods 336, 104–112 (2008).
Kaul, R., Johnson, K., Scholz, H. & Marr, G. Performance of the BioPlex 2200 autoimmune vasculitis kit. Autoimmun. Rev. 8, 224–227 (2009).
Nifli, A. P. et al. Comparison of a multiplex, bead-based fluorescent assay and immunofluorescence methods for the detection of ANA and ANCA autoantibodies in human serum. J. Immunol. Methods. 311, 189–197 (2006).
Mahler, M. et al. Development and performance evaluation of novel chemiluminescence assays for detection of anti-PR3 and anti-MPO antibodies. Clin. Chim. Acta 413, 719–726 (2012).
Ghillani, P. et al. Routine use of Zenit RA, a novel chemiluminescent immunoanalyzer in autoimmune disease diagnosis. Autoimmun. Highlights 3, 27–31 (2012).
Wiik, A. Delineation of a standard procedure for indirect immunofluorescence detection of ANCA. APMIS Suppl. 97 (Suppl. 6), 12–13 (1989).
Savige, J. et al. Addendum to the International Consensus Statement on testing and reporting of antineutrophil cytoplasmic antibodies. Quality control guidelines, comments, and recommendations for testing in other autoimmune diseases. Am. J. Clin. Pathol. 120, 312–318 (2003).
Monogioudi, E. et al. Development of a certified reference material for myeloperoxidase-antineutrophil cytoplasmic autoantibodies (MPO-ANCA). Clin. Chim. Acta 467, 48–50 (2017).
Selga, D., Segelmark, M., Gunnarsson, L. & Hellmark, T. Epitope shift of proteinase-3 anti-neutrophil cytoplasmic antibodies in patients with small vessel vasculitis. Clin. Exp. Immunol. 160, 318–324 (2010).
Silva, F., Hummel, A. M., Jenne, D. E. & Specks, U. Discrimination and variable impact of ANCA binding to different surface epitopes on proteinase 3, the Wegener's autoantigen. J. Autoimmun. 35, 299–308 (2010).
Van Der Geld, Y. M. et al. Antineutrophil cytoplasmic antibodies to proteinase 3 in Wegener's granulomatosis: epitope analysis using synthetic peptides. Kidney Int. 59, 147–159 (2001).
Roth, A. J. et al. Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J. Clin. Invest. 123, 1773–1783 (2013).
Gou, S. J., Xu, P. C., Chen, M. & Zhao, M. H. Epitope analysis of anti-myeloperoxidase antibodies in patients with ANCA-associated vasculitis. PLoS ONE. 8, e60530 (2013).
Suzuki, K. et al. Analysis of risk epitopes of anti-neutrophil antibody MPO-ANCA in vasculitis in Japanese population. Microbiol. Immunol. 51, 1215–1220 (2007).
Espy, C. et al. Sialylation levels of anti-proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener's). Arthritis Rheum. 63, 2105–2115 (2011).
Brouwer, E. et al. Predominance of IgG1 and IgG4 subclasses of anti-neutrophil cytoplasmic autoantibodies (ANCA) in patients with Wegener's granulomatosis and clinically related disorders. Clin. Exp. Immunol. 83, 379–386 (1991).
Kemna, M. J. et al. The avidity of PR3-ANCA in patients with granulomatosis with polyangiitis during follow-up. Clin. Exp. Immunol. 185, 141–147 (2016).
Gao, Y., Ye, H., Yu, F., Guo, X. H. & Zhao, M. H. Anti-myeloperoxidase IgG subclass distribution and avidity in sera from patients with propylthiouracil-induced antineutrophil cytoplasmic antibodies associated vasculitis. Clin. Immunol. 117, 87–93 (2005).
Mandl, L. A. et al. Using antineutrophil cytoplasmic antibody testing to diagnose vasculitis: can test-ordering guidelines improve diagnostic accuracy? Arch. Intern. Med. 162, 1509–1514 (2002).
Sinclair, D., Saas, M. & Stevens, J. M. The effect of a symptom related “gating policy” on ANCA requests in routine clinical practice. J. Clin. Pathol. 57, 131–134 (2004).
Arnold, D. F. et al. Does a gating policy for ANCA overlook patients with ANCA associated vasculitis? An audit of 263 patients. J. Clin. Pathol. 63, 678–680 (2010).
Bossuyt, X. et al. A multicentre study to improve clinical interpretation of proteinase-3 and myeloperoxidase anti-neutrophil cytoplasmic antibodies. Rheumatology (Oxford) 56, 1533–1541 (2017).
Vermeersch, P., Blockmans, D. & Bossuyt, X. Use of likelihood ratios can improve the clinical usefulness of enzyme immunoassays for the diagnosis of small-vessel vasculitis. Clin. Chem. 55, 1886–1888 (2009).
American College of Rheumatology ad hoc committee on immunologic testing guidelines. Guidelines for immunologic laboratory testing in the rheumatic diseases: an introduction. Arthritis Rheum. 47, 429–433 (2002).
Bossuyt, X. Clinical performance characteristics of a laboratory test. A practical approach in the autoimmune laboratory. Autoimmun. Rev. 8, 543–548 (2009).
Vermeersch, P. & Bossuyt, X. Comparative analysis of different approaches to report diagnostic accuracy. Arch. Intern. Med. 170, 734–735 (2010).
Langford, C. A. The diagnostic utility of C-ANCA in Wegener's granulomatosis. Cleve. Clin. J. Med. 65, 135–140 (1998).
Jennette, J. C., Wilkman, A. S. & Falk, R. J. Diagnostic predictive value of ANCA serology. Kidney Int. 53, 796–798 (1998).
Mouthon, L., Dunogue, B. & Guillevin, L. Diagnosis and classification of eosinophilic granulomatosis with polyangiitis (formerly named Churg-Strauss syndrome). J. Autoimmun. 48–49, 99–103 (2014).
Hellmark, T. & Segelmark, M. Diagnosis and classification of Goodpasture's disease (anti-GBM). J. Autoimmun. 48–49, 108–112 (2014).
Rutgers, A., Heeringa, P., Damoiseaux, J. G. & Tervaert, J. W. ANCA and anti-GBM antibodies in diagnosis and follow-up of vasculitic disease. Eur. J. Intern. Med. 14, 287–295 (2003).
Bossuyt, X. Serologic markers in inflammatory bowel disease. Clin. Chem. 52, 171–181 (2006).
Tervaert, J. W. et al. Antineutrophil cytoplasmic antibodies in primary sclerosing cholangitis, ulcerative colitis, and autoimmune diseases. Gastroenterology 102, 1090–1091 (1992).
Roozendaal, C. et al. Clinical significance of anti-neutrophil cytoplasmic antibodies (ANCA) in autoimmune liver diseases. J. Hepatol. 32, 734–741 (2000).
Dignass, A. et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J. Crohns Colitis 6, 965–990 (2012).
Mahler, M. et al. PR3-ANCA: a promising biomarker for ulcerative colitis with extensive disease. Clin. Chim. Acta 424, 267–273 (2013).
Arias-Loste, M. T. et al. Presence of anti-proteinase 3 antineutrophil cytoplasmic antibodies (anti-PR3 ANCA) as serologic markers in inflammatory bowel disease. Clin. Rev. Allergy Immunol. 45, 109–116 (2013).
Stinton, L. M. et al. PR3-ANCA: a promising biomarker in primary sclerosing cholangitis (PSC). PLoS ONE 9, e112877 (2014).
Sy, A. et al. Vasculitis in patients with inflammatory bowel diseases: a study of 32 patients and systematic review of the literature. Semin. Arthritis Rheum. 45, 475–482 (2016).
Humbert, S. et al. Inflammatory bowel diseases in anti-neutrophil cytoplasmic antibody-associated vasculitides: 11 retrospective cases from the French Vasculitis Study Group. Rheumatology (Oxford) 54, 1970–1975 (2015).
Weiner, M. & Segelmark, M. The clinical presentation and therapy of diseases related to anti-neutrophil cytoplasmic antibodies (ANCA). Autoimmun Rev. 15, 978–982 (2016).
Philipponnet, C. et al. Antineutrophilic cytoplasmic antibody-associated vasculitis and malignant hemopathies, a retrospective study of 16 cases. Joint Bone Spine. 84, 51–57 (2017).
Belizna, C. C., Hamidou, M. A., Levesque, H., Guillevin, L. & Shoenfeld, Y. Infection and vasculitis. Rheumatology (Oxford) 48, 475–482 (2009).
Langlois, V. et al. Antineutrophil cytoplasmic antibodies associated with infective endocarditis. Medicine (Baltimore) 95, e2564 (2016).
Mahr, A. et al. Brief report: prevalence of antineutrophil cytoplasmic antibodies in infective endocarditis. Arthritis Rheumatol. 66, 1672–1677 (2014).
Reza Ardalan, M. & Trillini, M. Infective endocarditis mimics ANCA associated glomerulonephritis. Caspian J. Intern. Med. 3, 496–499 (2012).
Ghosh, K., Pradhan, V. & Ghosh, K. Background noise of infection for using ANCA as a diagnostic tool for vasculitis in tropical and developing countries. Parasitol. Res. 102, 1093–1095 (2008).
Flores-Suárez, L. F., Cabiedes, J., Villa, A. R., van der Woude, F. J. & Alcocer-Varela, J. Prevalence of antineutrophil cytoplasmic autoantibodies in patients with tuberculosis. Rheumatology (Oxford) 42, 223–229 (2003).
Lima, I. et al. Anti-PR3 and anti-MPO antibodies are not present in sera of patients with pulmonary tuberculosis. Rheumatol. Int. 34, 1231–1234 (2014).
Grau, R. G. Drug-induced vasculitis: new insights and a changing lineup of suspects. Curr. Rheumatol. Rep. 17, 71 (2015).
Pendergraft, W. F. & Niles, J. L. Trojan horses: drug culprits associated with antineutrophil cytoplasmic autoantibody (ANCA) vasculitis. Curr. Opin. Rheumatol. 26, 42–49 (2014).
McGrath, M. M. et al. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated disease. Clin. J. Am. Soc. Nephrol. 6, 2799–2805 (2011).
Slot, M. C., Links, T. P., Stegeman, C. A. & Tervaert, J. W. Occurrence of antineutrophil cytoplasmic antibodies and associated vasculitis in patients with hyperthyroidism treated with antithyroid drugs: a long-term followup study. Arthritis Rheum. 53, 108–113 (2005).
Lenert, P., Icardi, M. & Dahmoush, L. ANA (+) ANCA (+) systemic vasculitis associated with the use of minocycline: case-based review. Clin. Rheumatol. 32, 1099–1106 (2013).
Gaffney, K. & Merry, P. Antineutrophil cytoplasmic antibody-positive polyarthritis associated with minocycline therapy. Br. J. Rheumatol. 35, 1327 (1996).
Dunphy, J., Oliver, M., Rands, A. L., Lovell, C. R. & McHugh, N. J. Antineutrophil cytoplasmic antibodies and HLA class II alleles in minocycline-induced lupus-like syndrome. Br. J. Dermatol. 142, 461–467 (2000).
Elkayam, O. et al. Clinical and immunological study of 7 patients with minocycline-induced autoimmune phenomena. Am. J. Med. 105, 484–487 (1998).
Author information
Authors and Affiliations
Contributions
J.D. and E.C. contributed equally to this work. X.B. and J.D. wrote the manuscript and researched the data for the article. All authors undertook review and/or editing of the manuscript before submission. X.B., J.W.C.T., L.F.F.-S., A.R., U.S., N.R., J.D. and E.C. provided substantial contributions to discussions of its content.
Corresponding author
Ethics declarations
Competing interests
XB has been a consultant for Inova Diagnostics and has received lecture fees from Inova Diagnostics, Menarini and Thermo Fisher. None of the other authors declare a competing interest.
Glossary
- Pre-test probability
-
Probability of an individual having a disease without prior knowledge of the results of laboratory tests.
- Likelihood ratios
-
The probability of a specific result occuring in a group of patients divided by the probability of the same result occuring in a group of controls.
- Post-test probability
-
Probability of an individual having a disease with prior knowledge of the results of laboratory tests.
- Odds
-
The conversion of probability to odds is carried out using the equation odds = probability/(1 − probability).
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bossuyt, X., Cohen Tervaert, JW., Arimura, Y. et al. Revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol 13, 683–692 (2017). https://doi.org/10.1038/nrrheum.2017.140
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2017.140
This article is cited by
-
Suitability of reduced dose glucocorticoids therapy regimen for antibody-associated vasculitis patients with TB: a retrospective study
European Journal of Clinical Microbiology & Infectious Diseases (2024)
-
Labordiagnostik bei Vaskulitiden jenseits von antineutrophilen zytoplasmatischen Autoantikörpern
Zeitschrift für Rheumatologie (2024)
-
Circulating cold-inducible RNA-binding protein levels in microscopic polyangiitis and granulomatosis with polyangiitis
Zeitschrift für Rheumatologie (2024)
-
Management der ANCA-assoziierten Vaskulitiden
Die Innere Medizin (2024)
-
Measurement of superoxide dismutase: clinical usefulness for patients with anti-neutrophil cytoplasmic antibody-associated vasculitis
Advances in Rheumatology (2023)