Key Points
-
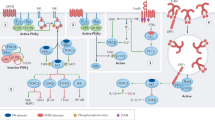
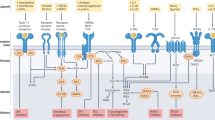
Dual-specificity phosphatases (DUSPs) are a subclass of protein tyrosine phosphatases (PTPs) that specifically interact with and regulate mitogen-activated protein kinases (MAPKs) by dephosphorylating phosphothreonine and tyrosine residues, thereby inactivating them. DUSPs can also regulate the subcellular localization of MAPKs, another important control point of their activity.
-
The three main MAPKs, extracellular signal-regulated kinase (ERK), p38 and c-Jun N-terminal kinase (JNK), have been intimately associated with immune responses, whereby the magnitude and duration of their activity determines the type of immune cell response, according to the type of stimuli they receive.
-
Approximately 16 DUSPs in the human genome have demonstrated activity towards MAPKs in vitro.
-
Individual DUSPs have different patterns of tissue expression, transcriptional regulation and subcellular localization, and have differing preferences for the MAPK subclasses that tightly modulate their activity.
-
Multiple inhibitors of the MAPK subclasses are in clinical trials for the treatment of inflammatory disorders. Important crosstalk and feedback loops within these pathways, as well as broad tissue expression profiles of MAPKs, may limit their success in the clinic.
-
Recent mouse knockout experiments have indicated for the first time individual, non-redundant functions for DUSPs in the regulation of cellular responses, particularly in inflammatory responses.
-
DUSPs have demonstrated both positive and negative control of immune responses such as sepsis, inflammatory arthritis and experimental autoimmune encephalomyelitis, which indicates that targeting DUSPs may be a viable approach for anti-inflammatory therapy.
Abstract
Dual-specificity phosphatases (DUSPs) are a subset of protein tyrosine phosphatases, many of which dephosphorylate threonine and tyrosine residues on mitogen-activated protein kinases (MAPKs), and hence are also referred to as MAPK phosphatases (MKPs). The regulated expression and activity of DUSP family members in different cells and tissues controls MAPK intensity and duration to determine the type of physiological response. For immune cells, DUSPs regulate responses in both positive and negative ways, and DUSP-deficient mice have been used to identify individual DUSPs as key regulators of immune responses. From a drug discovery perspective, DUSP family members are promising drug targets for manipulating MAPK-dependent immune responses in a cell-type and disease-context-dependent manner, to either boost or subdue immune responses in cancers, infectious diseases or inflammatory disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kumar, S., Boehm, J. & Lee, J. C. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nature Rev. Drug Discov. 2, 717–726 (2003).
O'Neill L, A. Targeting signal transduction as a strategy to treat inflammatory diseases. Nature Rev. Drug Discov. 5, 549–563 (2006).
Bissonnette, R. et al. A randomized, multicenter, double-blind, placebo-controlled phase 2 trial of ISA247 in patients with chronic plaque psoriasis. J. Am. Acad. Dermatol. 54, 472–478 (2006).
Abraham, S. M. et al. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J. Exp. Med. 203, 1883–1889 (2006).
Lasa, M., Abraham, S. M., Boucheron, C., Saklatvala, J. & Clark, A. R. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol. Cell. Biol. 22, 7802–7811 (2002).
Dong, C., Davis, R. J. & Flavell, R. A. MAP kinases in the immune response. Annu. Rev. Immunol. 20, 55–72 (2002).
Wada, T. & Penninger, J. M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23, 2838–2849 (2004).
Johnson, G. L. & Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298, 1911–1912 (2002).
Chang, L. & Karin, M. Mammalian MAP kinase signalling cascades. Nature 410, 37–40 (2001).
Fischer, A. M., Katayama, C. D., Pages, G., Pouyssegur, J. & Hedrick, S. M. The role of ERK1 and ERK2 in multiple stages of T cell development. Immunity 23, 431–443 (2005).
Crompton, T., Gilmour, K. C. & Owen, M. J. The MAP kinase pathway controls differentiation from double-negative to double-positive thymocyte. Cell 86, 243–251 (1996).
Behrens, A. et al. Jun N-terminal kinase 2 modulates thymocyte apoptosis and T cell activation through c-Jun and nuclear factor of activated T cell (NF-AT). Proc. Natl Acad. Sci. USA 98, 1769–1774 (2001).
Rincon, M. et al. The JNK pathway regulates the in vivo deletion of immature CD4+CD8+ thymocytes. J. Exp. Med. 188, 1817–1830 (1998).
Berenson, L. S., Yang, J., Sleckman, B. P., Murphy, T. L. & Murphy, K. M. Selective requirement of p38α MAPK in cytokine-dependent, but not antigen receptor-dependent, TH1 responses. J. Immunol. 176, 4616–4621 (2006).
Rincon, M., Flavell, R. A. & Davis, R. A. The JNK and p38 MAP kinase signaling pathways in T cell-mediated immune responses. Free Radic. Biol. Med. 28, 1328–1337 (2000).
Szabo, S. J., Sullivan, B. M., Peng, S. L. & Glimcher, L. H. Molecular mechanisms regulating TH1 immune responses. Annu. Rev. Immunol. 21, 713–758 (2003).
Merritt, C. et al. Activation of p38 mitogen-activated protein kinase in vivo selectively induces apoptosis of CD8+ but not CD4+ T cells. Mol. Cell. Biol. 20, 936–946 (2000).
Mahtani, K. R. et al. Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor α mRNA stability. Mol. Cell. Biol. 21, 6461–6469 (2001).
Dumitru, C. D. et al. TNF-α induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 103, 1071–1083 (2000). This is a pivotal paper describing the essential role of the TPL2–ERK pathway in TLR4 stimulation of macrophages for TNF post-translational stability by targeting the AU-rich element.
Lee, J. C. et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372, 739–746 (1994).
Park, J. M., Greten, F. R., Li, Z. W. & Karin, M. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science 297, 2048–2051 (2002).
Huang, C., Rajfur, Z., Borchers, C., Schaller, M. D. & Jacobson, K. JNK phosphorylates paxillin and regulates cell migration. Nature 424, 219–223 (2003).
Rousseau, S. et al. CXCL12 and C5a trigger cell migration via a PAK1/2–p38α MAPK–MAPKAP–K2–HSP27 pathway. Cell Signal. 18, 1897–1905 (2006).
Huang, C., Jacobson, K. & Schaller, M. D. MAP kinases and cell migration. J. Cell Sci. 117, 4619–4628 (2004).
Carragher, N. O. & Frame, M. C. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 14, 241–249 (2004).
Harada, H., Quearry, B., Ruiz-Vela, A. & Korsmeyer, S. J. Survival factor-induced extracellular signal-regulated kinase phosphorylates BIM, inhibiting its association with BAX and proapoptotic activity. Proc. Natl Acad. Sci. USA 101, 15313–15317 (2004).
Rui, L., Healy, J. I., Blasioli, J. & Goodnow, C. C. ERK signaling is a molecular switch integrating opposing inputs from B cell receptor and T cell cytokines to control TLR4-driven plasma cell differentiation. J. Immunol. 177, 5337–5346 (2006).
Reth, M. & Brummer, T. Feedback regulation of lymphocyte signalling. Nature Rev. Immunol. 4, 269–278 (2004).
Cheung, P. C., Campbell, D. G., Nebreda, A. R. & Cohen, P. Feedback control of the protein kinase TAK1 by SAPK2a/p38α. EMBO J. 22, 5793–5805 (2003). This paper shows the importance of feedback control of p38 and JNK and why inhibition of p38 may activate JNK pathways.
Friedman, A. & Perrimon, N. A functional RNAi screen for regulators of receptor tyrosine kinase and ERK signalling. Nature 444, 230–234 (2006).
Jeffrey, K. L. et al. Positive regulation of immune cell function and inflammatory responses by phosphatase PAC-1. Nature Immunol. 7, 274–283 (2006). The first description of DUSP2-deficient mice and positive regulation of immune responses by a DUSP. Also highlights the importance of in vivo analysis of DUSP proteins.
Murphy, L. O., Smith, S., Chen, R. H., Fingar, D. C. & Blenis, J. Molecular interpretation of ERK signal duration by immediate early gene products. Nature Cell Biol. 4, 556–564 (2002).
Volmat, V., Camps, M., Arkinstall, S., Pouyssegur, J. & Lenormand, P. The nucleus, a site for signal termination by sequestration and inactivation of p42/p44 MAP kinases. J. Cell Sci. 114, 3433–3443 (2001).
Pouyssegur, J., Volmot, V. & Lenormand, P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem. Pharm. 64, 755–763 (2002).
Harding, A., Tian, T., Westbury, E., Frische, E. & Hancock, J. F. Subcellular localization determines MAP kinase signal output. Curr. Biol. 15, 869–873 (2005).
Hazzalin, C. A. & Mahadevan, L. C. MAPK-regulated transcription: a continuously variable gene switch? Nature Rev. Mol. Cell Biol. 3, 30–40 (2002).
Zimmermann, S. et al. MEK1 mediates a positive feedback on Raf-1 activity independently of Ras and Src. Oncogene 15, 1503–1511 (1997).
Xiao, Y. Q. et al. Cross-talk between ERK and p38 MAPK mediates selective suppression of pro-inflammatory cytokines by transforming growth factor-β. J. Biol. Chem. 277, 14884–14893 (2002).
Shen, Y. H. et al. Cross-talk between JNK/SAPK and ERK/MAPK pathways: sustained activation of JNK blocks ERK activation by mitogenic factors. J. Biol. Chem. 278, 26715–26721 (2003).
Rommel, C. et al. Differentiation stage-specific inhibition of the Raf–MEK–ERK pathway by Akt. Science 286, 1738–1741 (1999).
Zimmermann, S. & Moelling, K. Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 286, 1741–1744 (1999).
Reth, M. Hydrogen peroxide as second messenger in lymphocyte activation. Nature Immunol. 3, 1129–1134 (2002).
Grumont, R. J., Rasko, J. E. J., Strasser, A., Gerondakis, S. Activation of the mitogen-activated protein kinase pathway induces transription of the PAC-1 phosphatase gene. Mol. Cell. Biol. 16, 2913–2921 (1996).
Camps, M. et al. Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science 280, 1262–1265 (1998). The first paper to describe the feedback control of DUSP catalytic activity by MAPKs.
Lin, Y. W. & Yang, J. L. Cooperation of ERK and SCFSkp2 for MKP-1 destruction provides a positive feedback regulation of proliferating signaling. J. Biol. Chem. 281, 915–926 (2006).
Brondello, J.-M., Brunet, A., Pouyssegur, J. & McKenzie, F. R. The dual specificity mitogen-activated protein kinase phosphatase-1 and-2 are induced by p42/p44 MAPK cascade. J. Biol. Chem. 272, 1368–1376 (1997).
Brondello, J. M., Pouyssegur, J. & McKenzie, F. R. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science 286, 2514–2517 (1999). Describes the control of DUSP protein stability by MAPKs.
Kamata, H. et al. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120, 649–661 (2005).
Salmeen, A. et al. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 423, 769–773 (2003).
Bhalla, U. S., Ram, P. T. & Iyengar, R. MAP kinase phosphatase as a locus of flexibility in a mitogen-activated protein kinase signaling network. Science 297, 1018–1023 (2002). A computational analysis highlighting the DUSPs as the control point of the MAPK signalling network.
Masuda, K., Shima, H., Watanabe, M. & Kikuchi, K. MKP-7, a novel mitogen-activated protein kinase phosphatase, functions as a shuttle protein. J. Biol. Chem. 276, 39002–39011 (2001).
Karlsson, M., Mathers, J., Dickinson, R. J., Mandl, M. & Keyse, S. M. Both nuclear-cytoplasmic shuttling of the dual specificity phosphatase MKP-3 and its ability to anchor MAP kinase in the cytoplasm are mediated by a conserved nuclear export signal. J. Biol. Chem. 279, 41882–41891 (2004). References 51 and 52 were the first to describe a role for DUSPs in controlling MAPK subcellular localization.
Canagarajah, B. J., Khokhlatchev, A., Cobb, M. H. & Goldsmith, E. J. Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell 90, 859–869 (1997).
Camps, M., Nichols, A. & Arkinstall, S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 14, 6–16 (2000).
Alonso, A. et al. Protein tyrosine phosphatases in the human genome. Cell 117, 699–711 (2004).
Andersen, J. N. et al. A genomic perspective on protein tyrosine phosphatases: gene structure, pseudogenes, and genetic disease linkage. FASEB J. 18, 8–30 (2004).
Lyon, M. A., Ducruet, A. P., Wipf, P. & Lazo, J. S. Dual-specificity phosphatases as targets for antineoplastic agents. Nature Rev. Drug Discov. 1, 961–976 (2002).
Keyse, S. M. Protein phosphatases and the regulation of MAP kinase activity. Semin. Cell Dev. Biol. 9, 143–152 (1998).
Tonks, N. K. & Neel, B. G. From form to function: signaling by protein tyrosine phosphatases. Cell 87, 365–368 (1996).
Tonks, N. K. & Neel, B. G. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr. Opin. Cell Biol. 13, 182–195 (2001).
Zhang, Y. et al. Regulation of innate and adaptive immune responses by MAP kinase phosphatase 5. Nature 430, 793–797 (2004). The first paper to describe a physiological role for a DUSP in vivo and a role for DUSPs in the immune system.
Chu, Y., Solski, P. A., Khosravi-Far, R., Der, C. J. & Kelly, K. The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J. Biol. Chem. 271, 6497–6501 (1996).
Ward, Y. et al. Control of MAP kinase activation by the mitogen-induced threonine/tyrosine phosphatase PAC1. Nature 367, 651–654 (1994).
Theodosiou, A. & Ashworth, A. MAP kinase phosphatases. Genome Biol. 3, 3009 (2002).
Keyse, S. M. An emerging family of dual specificity MAP kinase phosphatases. Biochim. Biophys. Acta 1265, 152–160 (1995).
Keyse, S. M. The role of protein phosphatases in the regulation of mitogen and stress-activated protein kinases. Free Radic. Res. 31, 341–349 (1999).
Keyse, S. M. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr. Opin. Cell Biol. 12, 186–192 (2000).
Ishibashi, T., Bottaro, D. P., Chan, A., Miki, T. & Aaronson, S. A. Expression cloning of a human dual-specificity phosphatase. Proc. Natl Acad. Sci. USA 89, 12170–12174 (1992).
Shin, D. Y. et al. A novel human ERK phosphatase regulates H-ras and v-raf signal transduction. Oncogene 14, 2633–2639 (1997).
Rahmouni, S. et al. Loss of the VHR dual-specific phosphatase causes cell-cycle arrest and senescence. Nature Cell Biol. 8, 524–531 (2006).
Zama, T. et al. Scaffold role of a mitogen-activated protein kinase phosphatase, SKRP1, for the JNK signaling pathway. J. Biol. Chem. 277, 23919–23926 (2002).
Zama, T. et al. A novel dual specificity phosphatase SKRP1 interacts with the MAPK kinase MKK7 and inactivates the JNK MAPK pathway. Implication for the precise regulation of the particular MAPK pathway. J. Biol. Chem. 277, 23909–23918 (2002).
Hammer, M. et al. Control of dual-specificity phosphatase-1 expression in activated macrophages by IL-10. Eur. J. Immunol. 35, 2991–3001 (2005).
Rohan, P. J. et al. PAC-1: A mitogen-induced nuclear protein tyrosine phosphatase. Science 259, 1763–1766 (1993).
Muda, M. et al. The dual specificity phosphatases M3/6 and MKP-3 are highly selective for inactivation of distinct mitogen-activated protein kinases. J. Biol. Chem. 271, 27205–27208 (1996).
Tanoue, T., Moriguchi, T. & Nishida, E. Molecular cloning and characterization of a novel dual specificity phosphatase, MKP-5. J. Biol. Chem. 274, 19949–19956 (1999).
Muda, M. et al. Molecular cloning and functional characterization of a novel mitogen-activated protein kinase phosphatase, MKP-4. J. Biol. Chem. 272, 5141–5151 (1997).
Christie, G. R. et al. The dual-specificity protein phosphatase DUSP9/MKP-4 is essential for placental function but is not required for normal embryonic development. Mol. Cell. Biol. 25, 8323–8333 (2005).
Tanzola, M. B. & Kersh, G. J. The dual specificity phosphatase transcriptome of the murine thymus. Mol. Immunol. 43, 754–762 (2006).
Dowd, S., Sneddon, A. A. & Keyse, S. M. Isolation of the human genes encoding the pyst1 and Pyst2 phosphatases: characterisation of Pyst2 as a cytosolic dual-specificity MAP kinase phosphatase and its catalytic activation by both MAP and SAP kinases. J. Cell Sci. 111, 3389–3399 (1998).
Camps, M. et al. Induction of the mitogen-activated protein kinase phosphatase MKP3 by nerve growth factor in differentiating PC12. FEBS Lett. 425, 271–276 (1998).
Keyse, S. M. & Emslie, E. A. Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature 359, 644–647 (1992).
Laderoute, K. R. et al. Mitogen-activated protein kinase phosphatase-1 (MKP-1) expression is induced by low oxygen conditions found in solid tumor microenvironments. A candidate MKP for the inactivation of hypoxia-inducible stress-activated protein kinase/c-Jun N-terminal protein kinase activity. J. Biol. Chem. 274, 12890–12897 (1999).
Seta, K. A., Kim, R., Kim, H. W., Millhorn, D. E. & Beitner-Johnson, D. Hypoxia-induced regulation of MAPK phosphatase-1 as identified by subtractive suppression hybridization and cDNA microarray analysis. J. Biol. Chem. 276, 44405–44412 (2001).
Groom, L. A., Sneddon, A. A., Alessi, D. R., Dowd, S. & Keyse, S. M. Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J. 15, 3621–3632 (1996).
Muda, M. et al. MKP-3, a novel cytosolic protein-tyrosine phosphatase that exemplifies a new class of mitogen-activated protein kinase phosphatase. J. Biol. Chem. 271, 4319–4326 (1996).
Fjeld, C. C., Rice, A. E., Kim, Y., Gee, K. R. & Denu, J. M. Mechanistic basis for catalytic activation of mitogen-activated protein kinase phosphatase 3 by extracellular signal-regulated kinase. J. Biol. Chem. 275, 6749–6757 (2000).
Slack, D. N., Seternes, O. M., Gabrielsen, M. & Keyse, S. M. Distinct binding determinants for ERK2/p38α and JNK map kinases mediate catalytic activation and substrate selectivity of map kinase phosphatase-1. J. Biol. Chem. 276, 16491–16500 (2001).
Farooq, A. et al. Solution structure of the MAPK phosphatase PAC-1 catalytic domain. insights into substrate-induced enzymatic activation of MKP. Structure 11, 155–164 (2003).
Zhang, Q. et al. New insights into the catalytic activation of the MAPK phosphatase PAC-1 induced by its substrate MAPK ERK2 binding. J. Mol. Biol. 354, 777–788 (2005).
Meng, T. C., Fukada, T. & Tonks, N. K. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell 9, 387–399 (2002). An important demonstration of catalytic inactivation of phosphatases by reversible oxidation.
Tanoue, T., Yamamoto, T., Maeda, R. & Nishida, E. A novel MAPK phosphatase MKP-7 acts preferentially on JNK/SAPK and p38α and β MAPKs. J. Biol. Chem. 276, 26629–26639 (2001).
Theodosiou, A., Smith, A., Gillieron, C., Arkinstall, S. & Ashworth, A. MKP5, a new member of the MAP kinase phosphatase family, which selectively dephosphorylates stress-activated kinases. Oncogene 18, 6981–6988 (1999).
Hirsch, D. D. & Stork, P. J. Mitogen-activated protein kinase phosphatases inactivate stress-activated protein kinase pathways in vivo. J. Biol. Chem. 272, 4568–4575 (1997).
Nichols, A. et al. Substrate recognition domains within extracellular signal-regulated kinase mediate binding and catalytic activation of mitogen-activated protein kinase phosphatase-3. J. Biol. Chem. 275, 24613–24621 (2000).
Hammer, M. et al. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J. Exp. Med. 203, 15–20 (2006).
Zhao, Q. et al. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J. Exp. Med. 203, 131–140 (2005). References 96 and 97 were 2 of 4 papers to come out over a 2-month period that described innate immune responses in DUSP1-deficient mice.
Rauhala, H. E. et al. Dual-specificity phosphatase 1 and serum/glucocorticoid-regulated kinase are downregulated in prostate cancer. Int. J. Cancer 117, 738–745 (2005).
Wang, H. Y., Cheng, Z. & Malbon, C. C. Overexpression of mitogen-activated protein kinase phosphatases MKP1, MKP2 in human breast cancer. Cancer Lett. 191, 229–237 (2003).
Xu, S., Furukawa, T., Kanai, N., Sunamura, M. & Horii, A. Abrogation of DUSP6 by hypermethylation in human pancreatic cancer. J. Hum. Genet. 50, 159–167 (2005).
Givant-Horwitz, V. et al. The PAC-1 dual specificity phosphatase predicts poor outcome in serous ovarian carcinoma. Gynecol. Oncol. 93, 517–523 (2004).
Kothapalli, R., Yoder, S. J., Kusmartseva, I. & Loughran, T. P. Jr. Characterization of a variant of PAC-1 in large granular lymphocyte leukemia. Protein Expr. Purif. 32, 52–60 (2003).
Levy-Nissenbaum, O. et al. Dual-specificity phosphatase Pyst2-L is constitutively highly expressed in myeloid leukemia and other malignant cells. Oncogene 22, 7649–7660 (2003).
Levy-Nissenbaum, O. et al. Overexpression of the dual-specificity MAPK phosphatase PYST2 in acute leukemia. Cancer Lett. 199, 185–192 (2003).
Yu, W. et al. A novel amplification target, DUSP26, promotes anaplastic thyroid cancer cell growth by inhibiting p38 MAPK activity. Oncogene 26, 1178–1187 (2006).
Li, M., Zhou, J. Y., Ge, Y., Matherly, L. H. & Wu, G. S. The phosphatase MKP1 is a transcriptional target of p53 involved in cell cycle regulation. J. Biol. Chem. 278, 41059–41068 (2003).
Yang, H. & Wu, G. S. p53 transactivates the phosphatase MKP1 through both intronic and exonic p53 responsive elements. Cancer Biol. Ther. 3, 1277–1282 (2004).
Dorfman, K. et al. Disruption of the erp/mkp-1 gene does not affect mouse development: normal MAP kinase activity in ERP/MKP-1-deficient fibroblasts. Oncogene 13, 925–931 (1996).
Zhao, Q. et al. The role of mitogen-activated protein kinase phosphatase-1 in the response of alveolar macrophages to lipopolysaccharide: attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. J. Biol. Chem. 280, 8101–8108 (2005).
Chi, H. et al. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc. Natl Acad. Sci. USA 103, 2274–2279 (2006).
Salojin, K. V. et al. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J. Immunol. 176, 1899–1907 (2006). References 110 and 111 were the other 2 of the 4 papers to come out over a 2-month period that described innate immune responses in DUSP1-deficient mice.
Wu, J. J. et al. Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metab. 4, 61–73 (2006).
Denu, J. M. & Dixon, J. E. Protein tyrosine phosphatases: mechanisms of catalysis and regulation. Curr. Opin. Chem. Biol. 2, 633–641 (1998).
Barford, D., Flint, A. J. & Tonks, N. K. Crystal structure of human protein tyrosine phosphatase 1B. Science 263, 1397–1404 (1994).
Farooq, A. et al. Solution structure of ERK2 binding domain of MAPK phosphatase MKP-3: structural insights into MKP-3 activation by ERK2. Mol. Cell 7, 387–399 (2001).
Lee, J. O. et al. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99, 323–334 (1999).
Tanoue, T., Yamamoto, T. & Nishida, E. Modular structure of a docking surface on MAPK phosphatases. J. Biol. Chem. 277, 22942–22949 (2002).
Pulido, R., Zuniga, A. & Ullrich, A. PTP-SL and STEP protein tyrosine phosphatases regulate the activation of the extracellular signal-regulated kinases ERK1 and ERK2 by association through a kinase interaction motif. EMBO J. 17, 7337–7350 (1998).
Tanoue, T. & Nishida, E. Molecular recognitions in the MAP kinase cascades. Cell Signal. 15, 455–462 (2003).
Zhao, Y. & Zhang, Z. Y. The mechanism of dephosphorylation of extracellular signal-regulated kinase 2 by mitogen-activated protein kinase phosphatase 3. J. Biol. Chem. 276, 32382–32391 (2001).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46, 3–26 (2001).
Bain, J., McLauchlan, H., Elliott, M. & Cohen, P. The specificities of protein kinase inhibitors: an update. Biochem. J. 371, 199–204 (2003).
Pages, G. et al. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science 286, 1374–1377 (1999).
Mazzucchelli, C. et al. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron 34, 807–820 (2002).
Yao, Y. et al. Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation. Proc. Natl Acad. Sci. USA 100, 12759–12764 (2003).
Hatano, N. et al. Essential role for ERK2 mitogen-activated protein kinase in placental development. Genes Cells 8, 847–856 (2003).
Adams, R. H. et al. Essential role of p38α MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell 6, 109–116 (2000).
Tamura, K. et al. Requirement for p38α in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell 102, 221–231 (2000).
Beardmore, V. A. et al. Generation and characterization of p38β (MAPK11) gene-targeted mice. Mol. Cell. Biol. 25, 10454–10464 (2005).
Sabio, G. et al. p38γ regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 24, 1134–1145 (2005).
Sabapathy, K. et al. JNK2 is required for efficient T-cell activation and apoptosis but not for normal lymphocyte development. Curr. Biol. 9, 116–125 (1999).
Dong, C. et al. Defective T cell differentiation in the absence of Jnk1. Science 282, 2092–2095 (1998).
Hirosumi, J. et al. A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 (2002).
Yang, D. D. et al. Differentiation of CD4+ T cells to TH1 cells requires MAP kinase JNK2. Immunity 9, 575–585 (1998).
Jaeschke, A. et al. Disruption of the Jnk2 (Mapk9) gene reduces destructive insulitis and diabetes in a mouse model of type I diabetes. Proc. Natl Acad. Sci. USA 102, 6931–6935 (2005).
Chen, A. J. et al. The dual specificity JKAP specifically activates the c-Jun N-terminal kinase pathway. J. Biol. Chem. 277, 36592–36601 (2002).
Caffrey, D. R., O'Neill, L. A. & Shields, D. C. The evolution of the MAP kinase pathways: coduplication of interacting proteins leads to new signaling cascades. J. Mol. Evol. 49, 567–582 (1999).
Roux, P. P. & Blenis, J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68, 320–344 (2004).
Bogoyevitch, M. A. & Court, N. W. Counting on mitogen-activated protein kinases — ERKs 3, 4, 5, 6, 7 and 8. Cell Signal. 16, 1345–1354 (2004).
Yoon, S. & Seger, R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 24, 21–44 (2006).
Robinson, M. J. & Cobb, M. H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 9, 180–186 (1997).
Pearson, G. et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22, 153–183 (2001).
Tonks, N. K. Protein tyrosine phosphatases: from genes, to function, to disease. Nature Rev. Mol. Cell Biol. 7, 833–846 (2006).
Charles, C. H., Sun, H., Lau, L. F. & Tonks, N. K. The growth factor-inducible immediate-early gene 3CH134 encodes a protein-tyrosine-phosphatase. Proc. Natl Acad. Sci. USA 90, 5292–5296 (1993).
Alessi, D. R., Smythe, C. & Keyse, S. M. The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene 8, 2015–2020 (1993).
Sun, H., Charles, C. H., Lau, L. F. & Tonks, N. K. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75, 487–493 (1993).
Manzano, R. G. et al. CL100 expression is down-regulated in advanced epithelial ovarian cancer and its re-expression decreases its malignant potential. Oncogene 21, 4435–4447 (2002).
Suzuki, C., Unoki, M. & Nakamura, Y. Identification and allelic frequencies of novel single-nucleotide polymorphisms in the DUSP1 and BTG1 genes. J. Hum. Genet. 46, 155–157 (2001).
Chattopadhyay, S. et al. MKP1/CL100 controls tumor growth and sensitivity to cisplatin in non-small-cell lung cancer. Oncogene 25, 3335–3345 (2006).
Vogt, A. et al. The benzo[c]phenanthridine alkaloid, sanguinarine, is a selective, cell-active inhibitor of mitogen-activated protein kinase phosphatase-1. J. Biol. Chem. 280, 19078–19086 (2005).
Lazo, J. S. et al. Novel benzofuran inhibitors of human mitogen-activated protein kinase phosphatase-1. Bioorg. Med. Chem. 14, 5643–5650 (2006).
Ueda, K. et al. 4-Isoavenaciolide covalently binds and inhibits VHR, a dual-specificity phosphatase. FEBS Lett. 525, 48–52 (2002).
Usui, T. et al. Design and synthesis of a dimeric derivative of RK-682 with increased inhibitory activity against VHR, a dual-specificity ERK phosphatase: implications for the molecular mechanism of the inhibition. Chem. Biol. 8, 1209–1220 (2001).
Guan, K. L. & Butch, E. Isolation and characterization of a novel dual specific phosphatase, HVH2, which selectively dephosphorylates the mitogen-activated protein kinase. J. Biol. Chem. 270, 7197–7203 (1995).
King, A. G., Ozanne, B. W., Smythe, C. & Ashworth, A. Isolation and characterisation of a uniquely regulated threonine, tyrosine phosphatase (TYP 1) which inactivates ERK2 and p54jnk. Oncogene 11, 2553–2563 (1995).
Ishibashi, T., Bottaro, D. P., Michieli, P., Kelley, C. A. & Aaronson, S. A. A novel dual specificity phosphatase induced by serum stimulation and heat shock. J. Biol. Chem. 269, 29897–29902 (1994).
Mandl, M., Slack, D. N. & Keyse, S. M. Specific inactivation and nuclear anchoring of extracellular signal-regulated kinase 2 by the inducible dual-specificity protein phosphatase DUSP5. Mol. Cell. Biol. 25, 1830–1845 (2005).
Mourey, R. J. et al. A novel cytoplasmic dual specificity protein tyrosine phosphatase implicated in muscle and neuronal differentiation. J. Biol. Chem. 271, 3795–3802 (1996).
Li, C., Scott, D. A., Hatch, E., Tian, X. & Mansour, S. L. Dusp6 (Mkp3) is a negative feedback regulator of FGF-stimulated ERK signaling during mouse development. Development 134, 167–176 (2007).
Vogt, A. et al. Cell-active dual specificity phosphatase inhibitors identified by high-content screening. Chem. Biol. 10, 733–742 (2003).
Theodosiou, A. M. et al. A member of the MAP kinase phosphatase gene family in mouse containing a complex trinucleotide repeat in the coding region. Hum. Mol. Genet. 5, 675–684 (1996).
Johnson, T. R., Biggs, J. R., Winbourn, S. E. & Kraft, A. S. Regulation of dual-specificity phosphatases M3/6 and hVH5 by phorbol esters. Analysis of a delta-like domain. J. Biol. Chem. 275, 31755–31762 (2000).
Masuda, K., Shima, H., Kikuchi, K., Watanabe, Y. & Matsuda, Y. Expression and comparative chromosomal mapping of MKP-5 genes DUSP10/Dusp10. Cytogenet. Cell Genet. 90, 71–74 (2000).
Muda, M., Manning, E. R., Orth, K. & Dixon, J. E. Identification of the human YVH1 protein-tyrosine phosphatase orthologue reveals a novel zinc binding domain essential for in vivo function. J. Biol. Chem. 274, 23991–23995 (1999).
Marti, F. et al. Negative-feedback regulation of CD28 costimulation by a novel mitogen-activated protein kinase phosphatase, MKP6. J. Immunol. 166, 197–206 (2001).
Wu, Q. et al. Dual specificity phosphotase 18, interacting with SAPK, dephosphorylates SAPK and inhibits SAPK/JNK signal pathway in vivo. Front. Biosci. 11, 2714–2724 (2006).
cShen, Y. et al. Activation of the Jnk signaling pathway by a dual-specificity phosphatase, JSP-1. Proc. Natl Acad. Sci. USA 98, 13613–13618 (2001).
Vasudevan, S. A. et al. MKP-8, a novel MAPK phosphatase that inhibits p38 kinase. Biochem. Biophys. Res. Commun. 330, 511–518 (2005).
Wang, J. Y., Lin, C. H., Yang, C. H., Tan, T. H. & Chen, Y. R. Biochemical and biological characterization of a neuroendocrine-associated phosphatase. J. Neurochem. 98, 89–101 (2006).
Acknowledgements
The authors would like to thank T. Brummer and R. Hooft van Huijsduijnen for critical evaluation of the manuscript, and S. Tange and K. Good for B-cell microarray data.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Development status of MAPK inhibitors for inflammation and cancer (PDF 198 kb)
Related links
Glossary
- Rheumatoid arthritis
-
A chronic, inflammatory autoimmune disorder in which leukocyte invasion of the synovial lining and hyperplasia of resident synoviocytes occurs. The ensuing overproduction of cytokines and other soluble mediators results in neovascularization, cartilage destruction, bone erosion and anarchic remodelling of joint structures.
- T-cell anergy
-
A state of T-cell unresponsiveness to stimulation with antigen. T-cell anergy can be induced by stimulation with a large amount of specific antigen in the absence of the engagement of co-stimulatory molecules.
- Thymocyte negative selection
-
The deletion of self-reactive thymocytes in the thymus. Thymocytes expressing T-cell receptors that strongly recognize self peptide bound to self MHC molecules undergo apoptosis in response to the signalling generated by high-affinity binding.
- Second-order reaction
-
A second-order reaction depends on the concentration of one second-order reactant or two first-order reactants. A kinase acting on a MAPK is a second-order reaction as it requires the MAPK and ATP (that is, two first-order reactants).
- Experimental autoimmune encephalomyelitis
-
An animal model of brain inflammation. It is mostly used with rodents and is a model for the human disease multiple sclerosis. It is induced with myelin oligodendrocyte glycoprotein.
Rights and permissions
About this article
Cite this article
Jeffrey, K., Camps, M., Rommel, C. et al. Targeting dual-specificity phosphatases: manipulating MAP kinase signalling and immune responses. Nat Rev Drug Discov 6, 391–403 (2007). https://doi.org/10.1038/nrd2289
Issue Date:
DOI: https://doi.org/10.1038/nrd2289
This article is cited by
-
Integrative function of histone deacetylase 3 in inflammation
Molecular Biology Reports (2024)
-
Combined KRAS-MAPK pathway inhibitors and HER2-directed drug conjugate is efficacious in pancreatic cancer
Nature Communications (2024)
-
Dual-specificity phosphatases 22-deficient T cells contribute to the pathogenesis of ankylosing spondylitis
BMC Medicine (2023)
-
ARID1A loss activates MAPK signaling via DUSP4 downregulation
Journal of Biomedical Science (2023)
-
ADSC secretome constrains NK cell activity by attenuating IL-2-mediated JAK-STAT and AKT signaling pathway via upregulation of CIS and DUSP4
Stem Cell Research & Therapy (2023)