Abstract
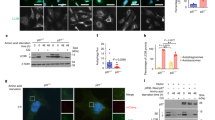
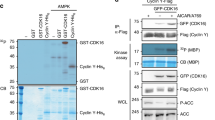
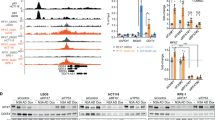
Nutrients and bioenergetics are prerequisites for proliferation and survival of mammalian cells1,2. We present evidence that the cyclin-dependent kinase inhibitor p27Kip1, is phosphorylated at Thr 198 downstream of the Peutz-Jeghers syndrome protein–AMP-activated protein kinase (LKB1–AMPK) energy-sensing pathway, thereby increasing p27 stability and directly linking sensing of nutrient concentration and bioenergetics to cell-cycle progression. Ectopic expression of wild-type and phosphomimetic Thr 198 to Asp 198 (T198D), but not unstable Thr 198 to Ala 198 (p27T198A) is sufficient to induce autophagy. Under stress conditions that activate the LKB1–AMPK pathway with subsequent induction of autophagy, p27 knockdown results in apoptosis. Thus LKB1–AMPK pathway-dependent phosphorylation of p27 at Thr 198 stabilizes p27 and permits cells to survive growth factor withdrawal and metabolic stress through autophagy. This may contribute to tumour-cell survival under conditions of growth factor deprivation, disrupted nutrient and energy metabolism, or during stress of chemotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pardee, A. A restriction point for control of normal animal cell proliferation. Proc. Natl Acad. Sci. USA 71, 1286–1290 (1974).
Hay, N. & Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 18, 1926–1945 (2004).
Wilson, W. & Roach, P. J. Nutrient-regulated protein kinases in budding yeast. Cell 111, 155–158 (2002).
Lum, J. et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 120, 237–248 (2005).
Inoki, K., Zhu, T. & Guan, K. L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577–590 (2003).
Hardie, D. The AMP-activated protein kinase pathway – new players upstream and downstream. J. Cell Sci. 117, 5479–5487 (2004).
Jones, R. et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 18, 283–293 (2005).
Kondo, Y., Kanzawa, T., Sawaya, R. & Kondo, S. The role of autophagy in cancer development and response to therapy. Nature Rev. Cancer 5, 726–734 (2005).
Lum, J., DeBerardinis, R. J. & Thompson, C.B. Autophagy in metazoans: cell survival in the land of plenty. Nature Rev. Mol. Cell Biol. 6, 439–448 (2005).
Reggiori, F. & Klionsky, D. J. Autophagosomes: biogenesis from scratch? Curr. Opin. Cell Biol. 17, 415–422 (2005).
Massagué, J. G1 cell-cycle control and cancer. Nature 432, 298–306 (2004).
Wu, F. et al. Reduction of cytosolic p27(Kip1) inhibits cancer cell motility, survival, and tumorigenicity. Cancer Res. 66, 2162–2172 (2006).
Slingerland, J. & Pagano, M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J. Cell Physiol. 183, 10–17 (2000).
Ishida, N., Kitagawa, M., Hatakeyama, S. & Nakayama, K. Phosphorylation at serine 10, a major phosphorylation site of p27(Kip1), increases its protein stability. J. Biol. Chem. 275, 25146–25154 (2000).
Kossatz, U. et al. C-terminal phosphorylation controls the stability and function of p27kip1. EMBO J. 25, 5159–5170 (2006).
Viglietto, G., Motti, M.L. & Fusco, A. Understanding p27Kip1 deregulation in cancer: down-regulation or mislocalization. Cell Cycle 1, 394–400 (2002).
Fujita, N., Sato, S. & Tsuruo, T. Phosphorylation of p27Kip1 at threonine 198 by p90 ribosomal protein S6 kinases promotes its binding to 14–3-3 and cytoplasmic localization. J. Biol. Chem. 278, 49254–49260 (2003).
Motti, M. et al. Complex regulation of the cyclin-dependent kinase inhibitor p27kip1 in thyroid cancer cells by the PI3K/AKT pathway: regulation of p27kip1 expression and localization. Am. J. Pathol. 166, 737–749 (2005).
Biederbick, A., Kern, H. F. & Elsasser, H.P. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur. J. Cell Biol. 66, 3–14 (1995).
Kabeya, Y. et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 (2000).
Takeuchi, H. et al. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 65, 3336–3346 (2005).
Eskelinen, E. et al. Inhibition of autophagy in mitotic animal cells. Traffic 3, 878–893 (2002).
Veneault-Fourrey, C., Barooah, M., Egan, M., Wakley, G. & Talbot, N. J. Autophagic fungal cell death is necessary for infection by the rice blast fungus. Science 312, 580–580 (2006).
Shaw, R. et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl Acad. Sci. USA 101, 3329–3335 (2004).
Lizcano, J. et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23, 833–843 (2004).
Michell, B. et al. Isoform-specific purification and substrate specificity of the 5'-AMP-activated protein kinase. Biol. Chem. 271, 28445–28450 (1996).
Motti, M., De Marco, C., Califano, D., Fusco, A. & Viglietto, G. Akt-dependent T198 phosphorylation of cyclin-dependent kinase inhibitor p27kip1 in breast cancer. Cell Cycle 3, 1074–1080 (2004).
Hahn-Windgassen, A. et al. Akt activates mTOR by regulating cellular ATP and AMPK activity. J. Biol. Chem. 3, 32081–32089 (2005).
Kiyokawa, H. et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 85, 721–732 (1996).
Levkau, B. et al. Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of Cdk2: role of a caspase cascade. Mol. Cell 1, 553–563 (1998).
Muraoka, R. et al. ErbB2/Neu-induced, cyclin D1-dependent transformation is accelerated in p27-haploinsufficient mammary epithelial cells but impaired in p27-null cells. Mol. Cell Biol. 22, 2204–2219 (2002).
Gao, H. et al. A critical role for p27kip1 gene dosage in a mouse model of prostate carcinogenesis. Proc. Natl Acad. Sci. USA 104, 17204–17209 (2004).
Liang, J. et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nature Med. 8, 1153–1160 (2002).
Lu, Y. et al. Src family protein-tyrosine kinases alter the function of PTEN to regulate phosphatidylinositol 3-kinase/AKT cascades. J. Biol. Chem. 278, 40057–40066 (2003).
Sheehan, K. et al. Use of reverse phase protein microarrays and reference standard development for molecular network analysis of metastatic ovarian carcinoma. Mol. Cell. Proteomics 4, 346–355 (2005).
Acknowledgements
J.L. is supported by the Odyssey Program of the Cockrell Foundation Award for Scientific Achievement from MD Anderson Cancer Center. We thank K. W. Cheng for help with statistical analysis, Y. Lu and Q. Yu for help with RPPA microarray, the MD Anderson Cancer Center Breast Tumor Bank for providing samples, and K. Donner Jr. for electron microscopy, and M.-C. Hung, S.-Y. Lin, F. X. Claret and J. R. Woodgett for helpful discussion. This work is supported by National Institutes of Health SPORE (P50-CA83639) and PO1 CA64602, PO1CA099031, CCSG grant P30 CA16672, and DAMD (17-02-01-0694) to G.B.M. and National Cancer Institue (CA63613), National Institute of Environmental Health Sciences (ES08263) and National Institute of Child Health and Human Development (HD046282) to C.L.W.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4 and S5 (PDF 895 kb)
Rights and permissions
About this article
Cite this article
Liang, J., Shao, S., Xu, ZX. et al. The energy sensing LKB1–AMPK pathway regulates p27kip1 phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol 9, 218–224 (2007). https://doi.org/10.1038/ncb1537
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1537
This article is cited by
-
RBM4 dictates ESCC cell fate switch from cellular senescence to glutamine-addiction survival through inhibiting LKB1-AMPK-axis
Signal Transduction and Targeted Therapy (2023)
-
New insights into activation and function of the AMPK
Nature Reviews Molecular Cell Biology (2023)
-
VEGFC ameliorates salt-sensitive hypertension and hypertensive nephropathy by inhibiting NLRP3 inflammasome via activating VEGFR3-AMPK dependent autophagy pathway
Cellular and Molecular Life Sciences (2023)
-
Nanosystem-mediated lactate modulation in the tumor micro environment for enhanced cancer therapy
Nano Research (2023)
-
Metformin and asarone inhibit HepG2 cell proliferation in a high glucose environment by regulating AMPK and Akt signaling pathway
Future Journal of Pharmaceutical Sciences (2021)