Abstract
Autosomal dominantly inherited spinocerebellar ataxias (SCAs) are a heterogeneous group of neurodegenerative disorders primarily affecting the cerebellum. Genetically, 26 different loci have been identified so far, although the corresponding gene has not yet been determined for 10 of them. Recently, mutations in the ATPase family gene 3-like 2 gene were presented to cause SCA type 28. To define the frequency of SCA28 mutations, we performed molecular genetic analyses in 140 unrelated familial cases with ataxia. Among other variations, we found a novel missense mutation at an evolutionarily conserved amino-acid position using a single-strand conformation polymorphism approach, followed by DNA sequencing. This amino-acid exchange p.E700K was detected in a four-generation German family and was not observed in a survey of 400 chromosomes from healthy control individuals.
Similar content being viewed by others
Introduction
Spinocerebellar ataxias (SCAs) with autosomal dominant inheritance are a clinically and genetically heterogeneous group of neurological disorders with overlapping and highly variable phenotypes characterized by progressive incoordination, dysarthria and impairment of eye movements. To date, 26 SCA loci have been identified by linkage analysis and, for at least 16, the respective gene has been determined. Nine SCAs are caused by expansion of tri- or pentanucleotide repeats, seven are due to deletions, missense, nonsense or frameshift mutations in the corresponding genes.1
In 2006, the locus for SCA type 28 (SCA28) was mapped to 18p11.22–q11.2 in a four-generation Italian family.2 After 2 years, the first set of SCA28 mutations was reported.3 At the annual meeting of the American Society of Human Genetics 2008, Cagnoli et al and Di Bella et al presented their results regarding the ATPase family gene 3-like 2 gene (AFG3L2) to be causative for SCA28. Overall, they have found at least six different missense mutations in the AFG3L2 gene in eight families. Affected individuals show slowly progressive gait and limb ataxia, dysarthria, hyperreflexia at lower limbs, nystagmus and ophthalmoparesis. Onset was reported to start at juvenile age (mean age: 27 years).
The AFG3L2 gene is composed of 17 coding exons. AFG3L2, the encoded protein, consists of 797 amino acids and exhibits different functional domains: an AAA consensus sequence together with an ATP/GTP-binding site, a peptidase M41 domain containing the HEXXH motif, which is a characteristic feature of a zinc-dependent binding domain, and an RNA-binding region.4 The ubiquitously expressed AFG3L2 is highly homologous to paraplegin, the product of the SPG7 gene. Mutations in the SPG7 gene are responsible for an autosomal recessive form of hereditary spastic paraplegia. Both AFG3L2 and paraplegin are metalloproteases that are components of the two mitochondrial AAA (m-AAA) protease isoenzymes in the inner mitochondrial membrane. The m-AAA heterocomplex is composed of both AFG3L2 and paraplegin, whereas the m-AAA homocomplex consists solely of AFG3L2. These proteases are known to exert chaperon-like activity and to participate in protein quality control. In addition, the AFG3L2 subunit functions as a processing enzyme for paraplegin, as well as for its own maturation.5
Different mouse models confirm the ataxia-inducing role of mutated AFG3L2, which primarily affects neuronal tissues. Afg3l2−/− genetic mutants lead to a severe ataxic phenotype with early lethality. In contrast, paraplegin-deficient mice show a mild form accompanied by late-onset axon degeneration.6 Furthermore, Spg7−/− mutant mice with a heterozygous mutation in the Afg3l2 gene are reported to be afflicted with an ataxic phenotype that is due to an accelerated axonopathy.7 Altogether, these data indicate the important role of AFG3L2 in neuronal function and a rather modulatory role of paraplegin.
A recently described mouse model, haploinsufficient for Afg3l2, exhibits several features common to the human SCA28 phenotype. Hence, this mouse model seems to be adequate to unravel the pathological cascade leading to this form of SCA.8
Notably, the six AFG3L2 mutations known so far occur in exons 15 and 16, both of which contribute to the peptidase M41 domain. All these missense exchanges hit evolutionarily conserved amino acids and none of them was found in 200 control chromosomes. To evaluate the frequency of AFG3L2 mutations among German ataxia cases, we screened a group of 140 unrelated patients with a familial history of dominant ataxia using a single-strand conformation polymorphism (SSCP) approach. With respect to the clustering of the described missense mutations, only the affected exons 15 and 16 were analysed. In addition, to check the SSCP results and to reveal intragenic variations, all 17 coding exons of the AFG3L2 gene were sequenced for 20 index patients.
Patients and methods
Subjects
A total of 140 unrelated German patients with familial ataxia and 200 healthy controls were studied. Before genetic analysis, mutations in the known SCA genes were excluded for the patients. After having obtained informed consent, genomic DNA was extracted from peripheral blood leukocytes by standard protocols.
SSCP analysis
PCR products of exons 15 and 16 were denatured at 95 °C for 5 min and immediately chilled on ice. The DNA strands were electrophoretically separated in a denaturing polyacrylamide gel (8% acrylamide and 10% urea) at 30 W for 4 hours at 25 °C. Subsequently, the gels were silver stained.
Sequence analysis
All 17 exons of the AFG3L2 gene were amplified by PCR. Fragment lengths were checked by electrophoretic separation on a 1.5% agarose gel. PCR products including exons as well as flanking intronic sequences were sequenced on both strands. Finally, sequences were analysed manually and computationally.
Allele-specific PCR
DNA samples from 200 unrelated control individuals of German descent were tested for the c.2098G>A exchange (p.E700K) by allele-specific PCR. Mutated and wild-type fragments were separated on a 2.5% agarose gel for 30 min. The primer sequences used were mut-forward 5′-ctgcaagattgatagatgata-3′, wt-forward 5′-cacgacgttgtaaaacgacctgcaagattgatagatgatg-3′, and wt-reverse 5′-ctgtgagaagagctactgttc-3′.
Accession codes (Ensembl release 54)
AFG3L2 Homo sapiens ENSG00000141385, AFG3L2 Homo sapiens transcript ENST00000269143, AFG3L2 Homo sapiens protein ENSP00000269143, Mus musculus ENSMUSP00000025408, Rattus norvegicus ENSRNOP00000024632, Canis familiaris ENSCAFP00000027762, Pongo pygmaeus ENSPPYP00000010085, Pan troglodytes ENSPTRP00000016808, Equus caballus ENSECAP00000004939 and Bos taurus ENSBTAP00000030993. Multiple sequence alignment was performed by using the online programme ‘CLUSTALW’ (http://align.genome.jp/). The clinical impact of the missense mutation p.E700K was assessed by the online version of ‘Mutation T@ster’ (http://www.mutationtaster.org/).
Clinical presentation
The first clinical signs in the male patient IV4 were noted at the age of  years and included impaired fine and gross motor skills and gait instability. MR imaging at that time confirmed cerebellar hypoplasia. Currently, at the age of
years and included impaired fine and gross motor skills and gait instability. MR imaging at that time confirmed cerebellar hypoplasia. Currently, at the age of  years, he attends elementary school and has problems with working speed, progredient difficulties in writing, as well as in fine motor skills. His neurological examination revealed ataxia, dysdiadochokinesia, muscular hypotonia and increased muscle reflexes. His 38-year-old mother, III9, reported first gait instability and clumsiness at the age of 10 years. When the patient was re-evaluated at the age of 36 years, she exhibited limb and gait ataxia, moderate dysarthria and bilateral gaze-evoked nystagmus, with normal reflexes and a lack of pyramidal signs. Further imaging studies (MRI) indicated cerebellar atrophy without any change in supratentorial compartments (including the corpus callosum) or in the brainstem. Despite clinical manifestation during infancy, the phenotype that is more than 25 years after onset is remarkably mild, allowing the patient to be completely independent.
years, he attends elementary school and has problems with working speed, progredient difficulties in writing, as well as in fine motor skills. His neurological examination revealed ataxia, dysdiadochokinesia, muscular hypotonia and increased muscle reflexes. His 38-year-old mother, III9, reported first gait instability and clumsiness at the age of 10 years. When the patient was re-evaluated at the age of 36 years, she exhibited limb and gait ataxia, moderate dysarthria and bilateral gaze-evoked nystagmus, with normal reflexes and a lack of pyramidal signs. Further imaging studies (MRI) indicated cerebellar atrophy without any change in supratentorial compartments (including the corpus callosum) or in the brainstem. Despite clinical manifestation during infancy, the phenotype that is more than 25 years after onset is remarkably mild, allowing the patient to be completely independent.
The 44-year-old cousin III3 first noted balance and writing disturbances after delivering her twin boys at the age of 28 years. She still works part time as an office employee, has mild dysarthria and currently needs to pay more attention during walking and talking. Re-evaluation at the age of 38 years revealed mild ataxia, dysdiadochokinesia, dysarthria, slight muscular hypotonia and increased muscle reflexes. Clinical findings are summarized in Table 1.
Results and discussion
In this study, DNA samples of 140 unrelated patients with a familial history of dominant ataxia were screened for sequence abnormalities in exons 15 and 16 of the AFG3L2 gene using an SSCP approach. Furthermore, to check the SSCP results and to reveal intragenic DNA sequence variations, all 17 coding exons were sequenced for 20 index patients. Altogether, we found five different single-nucleotide exchanges in the coding sequence, as well as in the 3′-UTR and intronic regions. One of these variations leads to a change at the amino-acid level (Table 2).
SSCP screening of 140 unrelated ataxia patients showed a peculiarity in exon 16 for one sample. Sequencing of this exon revealed the heterozygous transition c.2098G>A in a 44-year-old female patient from Germany (III3). At the amino-acid level, this single-nucleotide substitution results in the missense exchange p.E700K.
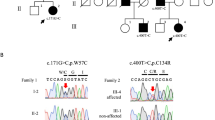
Remarkably, the variation c.2098G>A was not found in DNA samples of 200 unrelated control individuals of German descent by allele-specific PCR. Furthermore, the amino-acid substitution p.E700K segregated with the disease in four additional affected members of the four-generation family (Figure 1). In patient IV4, his mother III9 and in the twins IV1 and IV2, the first cerebellar signs of the disease developed within the first decade of life, whereas in five additional family members, symptoms were retrospectively noted within the third decade of life with an unusually slow progression and maintained free walking into the seventh decade of life. A more severe clinical presentation was only observed in both affected monozygotic twins, who in addition show a global developmental delay and currently attend a special school for children with cognitive impairment. However, given the normal cognitive function of the other affected relatives in this family and the few cases described so far in the literature, reported premature delivery and serious postnatal adaptation problems need to be considered as important cofactors for the more complex and severe clinical presentation of these monozygotic twins.
Pedigree of a four-generation German family with autosomal dominant spinocerebellar ataxia. Filled symbols indicate affected subjects. Open symbols specify unaffected spouses and presently asymptomatic family members. Deceased individuals are marked by a diagonal line. DNA samples of subjects III3, III9, IV1, IV2 and IV4 were available for molecular genetic analysis (arrows).
In contrast, predictive testing of one not affected family member revealed the wild-type sequence. On the basis of these findings, a pathogenic impact of the missense mutation p.E700K on the ataxic phenotype is highly presumable. This assumption is supported by the strong conservation of glutamic acid (E) at position 700 in M. musculus, R. norvegicus, C. familiaris, P. pygmaeus, P. troglodytes, E. caballus and B. taurus. Furthermore, the online programme ‘Mutation T@ster’ (http://www.mutationtaster.org/) classifies this amino-acid exchange as being presumably disease causing.
It is noteworthy that a pathogenic missense exchange E–K is already described in the literature.3 However, the authors do not specify the SCA28 gene name nor the exact position of this amino-acid substitution. Thus, it remains unclear whether both missense mutations are identical.
Subsequent to the SSCP screening, exons 15 and 16 were sequenced for 19 randomly selected individuals out of the 140 ataxia patients, as well as for the 44-year-old female patient III3 carrying the missense mutation p.E700K. No further DNA sequence abnormality was identified in any of the corresponding samples, indicating good reliability of the SSCP assay. Moreover, all remaining coding exons of the AFG3L2 gene were investigated by DNA sequence analysis for these 20 ataxia patients in order to reveal rare and underlying intragenic variations. This is due to the crucial necessity of gaining knowledge about the clinical impact of AFG3L2 variants to provide a firm basis for genetic testing in SCA28.
Single-nucleotide polymorphisms (SNPs) IVS7+6C>T and c.*28G>C were detected in intron 7 and in the 3′-UTR of the AFG3L2 gene, respectively. In addition to these noncoding SNPs, the two silent exchanges, p.L463 (exon 11) and p.E550 (exon 13), were found (Table 2). The intronic SNP and the two silent exchanges occur exclusively in the same individuals. Primarily, a pathogenic effect through impaired splicing would be possible for the IVS7+6C>T exchange because of its proximity to the exon/intron boundary. However, given the relatively high frequency of these four SNPs and the fact that no typical splice site is generated in all cases, a disease-causing impact through aberrant splicing is very unlikely for these variations.
Overall, only one ataxia-inducing mutation was found in 140 patients, illustrating that SCA28 is a relatively rare cause of disease in the German population. The neurological examination of the five affected family members further supports previous data regarding the clinical presentation of most SCA28 patients: an early-onset cerebellar ataxia with a slowly progressive phenotype. Notably, all AFG3L2 mutations known so far are located in a small interval between amino acids 654 and 700 in exons 15 and 16. Hence, for diagnostic purposes, investigating only these two exons should be taken into account. The clustering in the AFG3L2 peptidase M41 domain, as well as the missense nature of all described mutations, argues against haploinsufficiency as the pathogenetic mechanism. In fact, a dominant-negative effect through gain of function is more presumable.
References
Soong B, Paulson HL : Spinocerebellar ataxias: an update. Curr Opin Neurol 2007; 20: 438–446.
Cagnoli C, Mariotti C, Taroni F et al: SCA28, a novel form of autosomal dominant cerebellar ataxia on chromosome 18p11.22-q11.2. Brain 2006; 129: 235–242.
Mariotti C, Brusco A, Di Bella D et al: Spinocerebellar ataxia type 28: a novel autosomal dominant cerebellar ataxia characterized by slow progression and ophthalmoparesis. Cerebellum 2008; 7: 184–188.
Banfi S, Bassi MT, Andolfi G et al: Identification and characterization of AFG3L2, a novel paraplegin-related gene. Genomics 1999; 59: 51–58.
Koppen M, Bonn F, Ehses S, Langer T : Autocatalytic processing of m-AAA protease subunits in mitochondria. Mol Biol Cell 2009; 20: 4216–4224.
Maltecca F, Aghaie A, Schroeder DG et al: The mitochondrial protease AFG3L2 is essential for axonal development. J Neurosci 2008; 28: 2827–2836.
Martinelli P, La Mattina V, Bernacchia A et al: Genetic interaction between the m-AAA protease isoenzymes reveals novel roles in cerebellar degeneration. Hum Mol Genet 2009; 18: 2001–2013.
Maltecca F, Magnoni R, Cerri F, Cox GA, Quattrini A, Casari G : Haploinsufficiency of AFG3L2, the gene responsible for spinocerebellar ataxia type 28, causes mitochondria-mediated Purkinje cell dark degeneration. J Neurosci 2009; 29: 9244–9254.
Acknowledgements
We thank all patients for supplying blood samples for scientific research and their clinicians for collecting them. A part of this work was supported by the Deutsche Forschungsgemeinschaft (DFG: ZU 136/1-2) and by the German Heredo-Ataxia Society (DHAG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Edener, U., Wöllner, J., Hehr, U. et al. Early onset and slow progression of SCA28, a rare dominant ataxia in a large four-generation family with a novel AFG3L2 mutation. Eur J Hum Genet 18, 965–968 (2010). https://doi.org/10.1038/ejhg.2010.40
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2010.40
Keywords
This article is cited by
-
Multifaceted Roles of AFG3L2, a Mitochondrial ATPase in Relation to Neurological Disorders
Molecular Neurobiology (2023)
-
SCA28: Novel Mutation in the AFG3L2 Proteolytic Domain Causes a Mild Cerebellar Syndrome with Selective Type-1 Muscle Fiber Atrophy
The Cerebellum (2017)
-
Neurocognitive Characterization of an SCA28 Family Caused by a Novel AFG3L2 Gene Mutation
The Cerebellum (2017)
-
Mitochondrial Quality Control Proteases in Neuronal Welfare
Journal of Neuroimmune Pharmacology (2016)
-
Spinocerebellar ataxia 28: a novel AFG3L2 mutation in a German family with young onset, slow progression and saccadic slowing
Cerebellum & Ataxias (2015)