Abstract
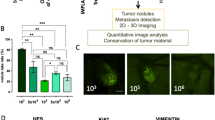
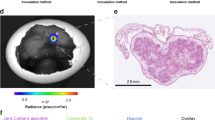
Oncolytic adenoviruses are promising anticancer agents. To study and optimize their tumor-killing potency, genuine tumor models are required. Here we describe the use of the chicken chorioallantoic membrane (CAM) tumor model in studies on oncolytic adenoviral vectors. Suspensions of human melanoma, colorectal carcinoma and glioblastoma multiforme cell lines were grafted on the CAM of embryonated chicken eggs. All cell lines tested formed 5–10 mm size tumors, which recapitulated hallmarks of corresponding human specimens. Furthermore, melanoma tumors were injected with adenoviral vector-carrying gene encoding the fusion protein of parainfluenza virus type 5. This led to the induction of cell fusion and syncytia formation in the infected cells. At 6 days post-injection, histological and immunohistochemical analyses of tumor sections confirmed adenovirus replication and syncytia formation. These results demonstrate that the CAM model allows rapid assessment of oncolytic viruses in three-dimensional tumors. Hence, this model constitutes an easy and affordable system for preclinical characterization of viral oncolytic agents that may precede the mandatory process of animal testing. Application of this model will help reducing the use of human xenografts in mice for preclinical evaluation of oncolytic viruses and other anticancer agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Okegawa T, Pong RC, Li Y, Bergelson JM, Sagalowsky AI, Hsieh JT . The mechanism of the growth-inhibitory effect of coxsackie and adenovirus receptor (CAR) on human bladder cancer: a functional analysis of car protein structure. Cancer Res 2001; 61: 6592–6600.
Kuppen PJ, van der Eb MM, Jonges LE, Hagenaars M, Hokland ME, Nannmark U et al. Tumor structure and extracellular matrix as a possible barrier for therapeutic approaches using immune cells or adenoviruses in colorectal cancer. Histochem Cell Biol 2001; 115: 67–72.
Harrison D, Sauthoff H, Heitner S, Jagirdar J, Rom WN, Hay JG . Wild-type adenovirus decreases tumor xenograft growth, but despite viral persistence complete tumor responses are rarely achieved—deletion of the viral E1b-19-kD gene increases the viral oncolytic effect. Hum Gene Ther 2001; 12: 1323–1332.
Sauthoff H, Hu J, Maca C, Goldman M, Heitner S, Yee H et al. Intratumoral spread of wild-type adenovirus is limited after local injection of human xenograft tumors: virus persists and spreads systemically at late time points. Hum Gene Ther 2003; 14: 425–433.
Worgall S, Wolff G, Falck-Pedersen E, Crystal RG . Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther 1997; 8: 37–44.
Ganesh S, Gonzalez Edick M, Idamakanti N, Abramova M, Vanroey M, Robinson M et al. Relaxin-expressing, fiber chimeric oncolytic adenovirus prolongs survival of tumor-bearing mice. Cancer Res 2007; 67: 4399–4407.
Kim J-H, Lee Y-S, Kim H, Huang J-H, Yoon AR, Yun C-O . Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J Natl Cancer Inst 2006; 98: 1482–1493.
Gros A, Martínez-Quintanilla J, Puig C, Guedan S, Molleví DG, Alemany R et al. Bioselection of a gain of function mutation that enhances adenovirus 5 release and improves its antitumoral potency. Cancer Res 2008; 68: 8928–8937.
Norrby K . In vivo models of angiogenesis. J Cell Mol Med 2006; 10: 588–612.
Hallak LK, Merchan JR, Storgard CM, Loftus JC, Russell SJ . Targeted measles virus vector displaying echistatin infects endothelial cells via alpha(v)beta3 and leads to tumor regression. Cancer Res 2005; 65: 5292–5300.
Ong HT, Trejo TR, Pham LD, Oberg AL, Russell SJ, Peng KW . Intravascularly administered RGD-displaying measles viruses bind to and infect neovessel endothelial cells in vivo. Mol Ther 2009; 17: 1012–1021.
Kim J, Yu W, Kovalski K, Ossowski L . Requirement for specific proteases in cancer cell intravasation as revealed by a novel semiquantitative PCR-based assay. Cell 1998; 94: 353–362.
Lugassy C, Kleinman HK, Engbring JA, Welch DR, Harms JF, Rufner R et al. Pericyte-like location of GFP-tagged melanoma cells: ex vivo and in vivo studies of extravascular migratory metastasis. Am J Pathol 2004; 164: 1191–1198.
Lugassy C, Vernon SE, Busam K, Engbring JA, Welch DR, Poulos EG et al. Angiotropism of human melanoma: studies involving in transit and other cutaneous metastases and the chicken chorioallantoic membrane: implications for extravascular melanoma invasion and metastasis. Am J Dermatopathol 2006; 28: 187–193.
Zijlstra A, Mellor R, Panzarella G, Aimes RT, Hooper JD, Marchenko ND et al. A quantitative analysis of rate-limiting steps in the metastatic cascade using human-specific real-time polymerase chain reaction. Cancer Res 2002; 62: 7083–7092.
Hagedorn M, Javerzat S, Gilges D, Meyre A, de Lafarge B, Eichmann A et al. Accessing key steps of human tumor progression in vivo by using an avian embryo model. Proc Natl Acad Sci USA 2005; 102: 1643–1648.
Taizi M, Deutsch VR, Leitner A, Ohana A, Goldstein RS . A novel and rapid in vivo system for testing therapeutics on human leukemias. Exp Hematol 2006; 34: 1698–1708.
Kobayashi T, Koshida K, Endo Y, Imao T, Uchibayashi T, Sasaki T et al. A chick embryo model for metastatic human prostate cancer. Eur Urol 1998; 34: 154–160.
Guedan S, Gros A, Cascallo M, Vile R, Mercade E, Alemany R . Syncytia formation affects the yield and cytotoxicity of an adenovirus expressing a fusogenic glycoprotein at a late stage of replication. Gene Therapy 2008; 15: 1240–1245.
Li H, Haviv YS, Derdeyn CA, Lam J, Coolidge C, Hunter E et al. Human immunodeficiency virus type 1-mediated syncytium formation is compatible with adenovirus replication and facilitates efficient dispersion of viral gene products and de novo-synthesized virus particles. Hum Gene Ther 2001; 12: 2155–2165.
Terrier O, Durupt F, Cartet G, Thomas L, Lina B, Rosa-Calatrava M . Engineering of a parainfluenza virus type 5 fusion protein (PIV-5 F): development of an autonomous and hyperfusogenic protein by a combinational mutagenesis approach. Virus Res 2009; 146: 115–124.
Mittereder N, March KL, Trapnell BC . Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol 1996; 70: 7498–7509.
Fallaux FJ, Bout A, van der Velde I, van den Wollenberg DJ, Hehir KM, Keegan J et al. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum Gene Ther 1998; 9: 1909–1917.
Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA . Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol 2010; 148: 3–15.
Damia G, D’Incalci M . Contemporary pre-clinical development of anticancer agents—what are the optimal preclinical models? Eur J Cancer 2009; 45: 2768–2781.
Morton CL, Houghton PJ . Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc 2007; 2: 247–250.
Candolfi M, Curtin JF, Nichols WS, Muhammad AG, King GD, Pluhar GE et al. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol 2007; 85: 133–148.
Lam JT, Hemminki A, Kanerva A, Lee KB, Blackwell JL, Desmond R et al. A three-dimensional assay for measurement of viral-induced oncolysis. Cancer Gene Ther 2007; 14: 421–430.
Lopez MV, Viale DL, Cafferata EG, Bravo AI, Carbone C, Gould D et al. Tumor associated stromal cells play a critical role on the outcome of the oncolytic efficacy of conditionally replicative adenoviruses. PLoS One 2009; 4: e5119.
Ahmed A, Jevremovic D, Suzuki K, Kottke T, Thompson J, Emery S et al. Intratumoral expression of a fusogenic membrane glycoprotein enhances the efficacy of replicating adenovirus therapy. Gene Therapy 2003; 10: 1663–1671.
Bateman A, Bullough F, Murphy S, Emiliusen L, Lavillette D, Cosset FL et al. Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumor growth. Cancer Res 2000; 60: 1492–1497.
Lin EH, Salon C, Brambilla E, Lavillette D, Szecsi J, Cosset FL et al. Fusogenic membrane glycoproteins induce syncytia formation and death in vitro and in vivo: a potential therapy agent for lung cancer. Cancer Gene Ther 2010; 17: 256–265.
Lamfers MLM, Gianni D, Tung C-H, Idema S, Schagen FHE, Carette JE et al. Tissue inhibitor of metalloproteinase-3 expression from an oncolytic adenovirus inhibits matrix metalloproteinase activity in vivo without affecting antitumor efficacy in malignant glioma. Cancer Res 2005; 65: 9398–9405.
Fang L, Pu Y-Y, Hu X-C, Sun L-J, Luo H-M, Pan S-K et al. Antiangiogenesis gene armed tumor-targeting adenovirus yields multiple antitumor activities in human HCC xenografts in nude mice. Hepatol Res 2010; 40: 216–228.
He X-P, Su C-Q, Wang X-H, Pan X, Tu Z-X, Gong Y-F et al. E1B-55kD-deleted oncolytic adenovirus armed with canstatin gene yields an enhanced anti-tumor efficacy on pancreatic cancer. Cancer Lett 2009; 285: 89–98.
Yoo JY, Kim J-H, Kwon Y-G, Kim E-C, Kim NK, Choi HJ et al. VEGF-specific short hairpin RNA-expressing oncolytic adenovirus elicits potent inhibition of angiogenesis and tumor growth. Mol Ther: J Am Soc Gene Ther 2007; 15: 295–302.
Zhang Z, Zou W, Wang J, Gu J, Dang Y, Li B et al. Suppression of tumor growth by oncolytic adenovirus-mediated delivery of an antiangiogenic gene, soluble Flt-1. Mol Ther: J Am Soc Gene Ther 2005; 11: 553–562.
Acknowledgements
We thank B Bancel for precious technical assistance in immunohistochemistry, L Fanchi for his assistance with photography and Drs L Depaepe, F Ragage, S Isaac and A Vasiljevic for providing the human samples and for their help with interpreting histological sections. FD was supported by the Fondation René Touraine (France) and the Institut Servier (France), RCH and DKL were supported by the European Union through the 6th Framework Program GIANT (Contract No. 512087) and MRC was supported by a Contrat d’Interface grant from the Hospices Civils de Lyon (France) and by a grant from La Ligue Contre le Cancer (France).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Durupt, F., Koppers-Lalic, D., Balme, B. et al. The chicken chorioallantoic membrane tumor assay as model for qualitative testing of oncolytic adenoviruses. Cancer Gene Ther 19, 58–68 (2012). https://doi.org/10.1038/cgt.2011.68
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2011.68
Keywords
This article is cited by
-
Efficient gene delivery into the embryonic chicken brain using neuron-specific promoters and in ovo electroporation
BMC Biotechnology (2022)
-
Chick chorioallantoic membrane assay as an in vivo model to study the effect of nanoparticle-based anticancer drugs in ovarian cancer
Scientific Reports (2018)
-
Natural products against cancer angiogenesis
Tumor Biology (2016)
-
Identification of flubendazole as potential anti-neuroblastoma compound in a large cell line screen
Scientific Reports (2015)
-
Imaging preclinical tumour models: improving translational power
Nature Reviews Cancer (2014)