Abstract
Background:
There are neither prospective data nor agreement on the optimal routine follow-up procedures in patients treated for soft tissue sarcoma of the limb.
Methods:
Data on 174 consecutive patients with a soft tissue sarcoma of the limb undergoing follow-up by oncologists at a single centre from 2003 to 2009 were included in this analysis. The rate and site of recurrence and mode of detection were analysed. Outcome of the patients was assessed.
Results:
Eighty-two patients (47%) experienced relapse of any type. Isolated local recurrence occurred in 26 patients and local relapse with synchronous pulmonary metastases in five patients. Local recurrences were detected clinically in 30 of these 31 patients; magnetic resonance imaging identified only one local recurrence. Twenty-eight patients developed isolated lung metastases; in nine patients these were amenable to resections, seven of whom are currently free of disease after treatment. Lung metastases were detected by chest x-ray (CXR) in 19 patients, computed tomography scanning in 3 patients, and clinically in 11 patients. Twenty-three patients developed non-pulmonary metastases. More than 80% of relapses occurred in the first 2 years of follow-up; however, later recurrences were also observed.
Conclusions:
Routine follow-up CXR can detect lung metastases suitable for surgical resection, although the optimal interval of imaging has yet to be defined. Local relapse is almost always detected by patients or physicians, and routine scanning of the primary site is of doubtful benefit. Patient and physician education to detect local relapse may be helpful. Prospective evaluation of follow-up is recommended.
Similar content being viewed by others
Main
Sarcomas are a rare and heterogeneous group of malignant tumours of mesenchymal origin. They account for <1% of all new cancer cases diagnosed in the UK each year. The number of new cases diagnosed in England between 2000 and 2009 was 2626 (National Cancer Intelligence Network, 2012). Approximately 80% of all sarcomas arise from soft tissue and the remaining from bone. Soft tissue sarcomas (STS) most frequently originate within an extremity (70%), followed by the trunk, retroperitoneum, and head and neck (Zagars et al, 2003). The standard treatment of localised STS of the extremities is limb-preserving surgery (Rosenberg et al, 1982; Potter et al, 1986), frequently in combination with adjuvant or neoadjuvant radiotherapy in intermediate- and high-grade disease (Barkley et al, 1988; Wilson et al, 1994; Wanebo et al, 1995; Yang et al, 1998; O'Sullivan et al, 2002). This combined modality treatment achieves 5-year local control rates of 80–90% and 5-year disease-specific survival rates of >70% (Zagars et al, 2003). Local recurrences have been documented to mostly occur up to 5 years after completion of treatment (Ballo et al, 2004), and in synovial sarcomas metastatic relapse has been shown to occur as late as 16.3 years after primary treatment (Krieg et al, 2011). A recent analysis of outcome in intermediate- and high-grade myxofibrosaromas showed local recurrences in 31% of patients and distant recurrences in 17%, indicating a more aggressive local behaviour compared with other STS (Haglund et al, 2012).
Surveillance strategies in cancer are adopted for the following: detection of local or distant recurrences based on the supposition that early detection and treatment prolongs survival; detection of secondary tumours or other relevant medical conditions, which might have been caused by previous anti-cancer treatment; and as a source of reassurance to the patient. However, there is concern about the negative effects of using excessive radiation in surveillance imaging of cancer survivors (Brenner and Hall, 2007; Tarin et al, 2009) and about causing anxiety by frequent investigations (Thompson et al, 2010).
There have been few prospective studies evaluating follow-up strategies in common cancer types, and none in STS. In breast cancer, survival has been shown to be equivalent with either intensive or routine follow-up (Palli et al, 1999). In stage I non-seminomatous germ cell tumours, the number of computed tomography (CT) scans during post-treatment surveillance was prospectively assessed and could be safely reduced from five to two with equivalent patient outcomes (Rustin et al, 2007). Treating patients with recurrent ovarian cancer on the basis of a rise in CA125 levels alone did not achieve superior outcomes when compared with deferring treatment to symptomatic relapse (Rustin et al, 2010). In colorectal cancer there is evidence that follow-up programmes for patients with curatively resected tumours improve survival, although which tests or frequencies of visits are optimal remains unclear (Figueredo et al, 2003). Recent data on surveillance in diffuse large B-cell lymphoma demonstrated that scanning detected diffuse large B-cell lymphoma relapse prior to clinical manifestations in only 8 out of 537 patients (1.5%) (Thompson et al, 2013).
Because of the lack of prospective data, follow-up recommendations for STS after primary curative treatment are based on expert opinions and vary in frequency and modality. All include regular chest imaging and some include routine imaging of the primary site (Grimer et al, 2010; ESMO/European Sarcoma Network Working Group, 2012; NCCN, 2014). A 2004 survey in UK cancer centres illustrates wide variations in follow-up practices, with little consistency on imaging, follow-up intervals, or duration of follow-up (Gerrand et al, 2007). An ideal surveillance schedule should meet the criteria of easy implementation, efficiency in detection of recurrence, and cost effectiveness.
The aim of this study was to describe the presentation of relapse, the modality of detection of recurrence, and patient outcomes, and to assess the effectiveness of current unit follow-up guidelines. We retrospectively assessed all patients who presented with STS of the limb between December 2003 and December 2009 at a large regional specialist non-surgical sarcoma unit who had undergone resection of the primary tumour. Most patients were referred for evaluation for radiotherapy and chemotherapy, thus representing a high-risk group of patients.
Patients and Methods
This retrospective analysis includes data on 174 patients with localised STS of the limbs who presented as new patients at the London Sarcoma Service at University College London Hospital (UCLH) between December 2003 and December 2009. Patient and tumour data were collected from hospital records.
Follow-up guidelines (Table 1) were implemented within the London Sarcoma Service in April 2007; previously, post-treatment surveillance was not standardised and was performed at the discretion of the treating physician. However, follow-up practice did not change substantially in the period prior to guideline implementation.
The median follow-up duration of all patients was 32 (range 1–91) months. For patients still alive median follow-up was 37 (range 1–91) months, and for those who had died it was 18 (range 7–70) months.
Information retrieved included age at surgery, tumour site, histological subtype, histological tumour grade (Coindre et al, 1988), treatment modalities, date of recurrence, location of recurrence (local, local and lung, lung only, non-pulmonary), mode of detection of relapse, and treatment for recurrence. Time to relapse was calculated from the date of completion of treatment to the date of relapse, with the exception of patients who relapsed following surgery and prior to starting adjuvant radiotherapy, when date of surgery to the date of relapse was used. The method of Kaplan and Meier was used for relapse-free survival analysis (Kaplan, 1958). Statistical calculations were performed with R version 3.0.1 (CORE TEAM R, 2013) and confidence intervals (CI) for median survival were calculated (Brookmeyer and Crawley, 1982).
Data collection was in accordance with local ethical standards.
Results
In total, 174 patients were assessed. Patient and tumour characteristics are summarised in Table 2.
Of the 174 patients, 172 underwent surgery; two patients received definitive combination chemo-radiation and underwent no surgery. The mean age at surgery was 51 (range 13–88) years. Radiotherapy was delivered to 145 of the 174 patients (83%) in addition to surgery: 25 patients received preoperative radiotherapy, and 118 patients received postoperative radiotherapy; two patients received pre- and postoperative radiotherapy. Twenty-five (14%) patients received neo- or adjuvant chemotherapy.
At the time of last follow-up, 116 patients (67%) were alive, 43 patients (25%) had died, and 15 (9%) were lost to follow-up (as defined by no follow-up information within the last 12 months). At the time of last follow-up, 112 patients (64%) were disease free.
Site of relapse
Tumour recurrence was experienced by 82 patients (47%). Sites of relapse are summarised in Table 3. Among patients who received radiotherapy (147 patients), the overall relapse rate was 42%, with a local relapse rate of 14%. In patients treated with surgery alone (27 patients), the overall relapse rate was 78%, with a local relapse rate of 37%.
Time of relapse
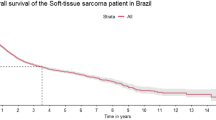
The median relapse-free survival for the entire group was 43.9 months (95% CI, 29.9 – infinity) (Figure 1). Disease-free survival at 2 years was 61% (95% CI 53–68) and that at 5 years was 42% (95% CI 32–51). Local relapse-free survival at 2 years was 75% (95% CI 67–81) and that at 5 years was 51% (95% CI 39–62). Median time from end of treatment to relapse was 9 (range 0–49) months for local recurrence, 3 (range 0.6–15) months for local relapse and synchronous lung metastases, 5 (range 1–66) months for lung metastases only, and 11 (range 3–52) months for non-pulmonary metastases (Figure 2). In some of these patients local as well as systemic relapse occurred prior to initiation of adjuvant radiotherapy.
During the first year 48 (59%) of 82 patients relapsed (13 local, 4 local with synchronous lung metastases, 18 lung relapses only), and a further 18 patients relapsed during the second year of follow-up (6 local, 1 local with synchronous lung metastases, and 5 lung recurrences). There was one relapse beyond 5 years; however, median follow-up was only 32 months.
Detection of relapse
Local relapse
Thirty of 31 local recurrences (97%) were detected clinically. In 22 patients detection was by self-examination, and in 8 cases recurrence was noted on physical examination by the physician at routine follow-up. Only one patient had an asymptomatic local relapse detected on magnetic resonance imaging (MRI), when repeat imaging was performed to reassess a postoperative seroma.
Our current practice is to routinely perform an MRI of the primary tumour site 6 months after completion of adjuvant radiotherapy, to act as a post-treatment baseline for future comparison. However, some patients underwent additional scans routinely for local surveillance. In 16 patients with a local relapse who had had a previous MRI not suspicious for recurrence, the median time from MRI to clinical detection of relapse was 6 (range 3–19) months. This indicates that, despite recent normal imaging, local recurrences were still detected clinically in this group of patients. The median time from previous normal follow-up to local relapse was 3 (range 1–28) months, which highlights the fact that local relapses were noted between regular 3-monthly appointments by the patient in the majority of cases.
Lung metastases
Twenty-eight patients (16%) developed isolated lung metastases. Asymptomatic lung metastases were discovered on routine chest X-ray (CXR) in 19 patients and by CT scanning in 3 patients at a median of 8 (range 1–66) months after completion of initial treatment. Of the three patients who had CT scans, one had indeterminate lung nodules on an original staging CT scan at first diagnosis, and a repeat CT scan after 3 months confirmed progression of the nodules, thereby diagnosing lung metastases; two patients underwent CT scans for other reasons (to exclude an axillary nodal recurrence; to reassess pelvic lymph nodes that had been irradiated as part of primary treatment), and the lung metastases were an incidental finding. As such, none of these three patients represent a deviation from our defined follow-up guidelines. Symptomatic lung metastases occurred in 6 patients at 2, 3, 5, 5, 5, and 40 months after the end of primary sarcoma treatment (median 5 months). The short time from initial treatment to symptomatic relapse in five of these patients suggests aggressive tumour biology. Relapse was detected at a median of 2 (range 1–5) months after the last routine follow-up and normal imaging. The patient with disease recurrence more than 3 years after surgery developed symptoms of a pleural effusion secondary to lung metastases, <3 months after a routine follow-up appointment.
Non-pulmonary metastases
Eighteen of 23 non-pulmonary recurrences (lymph node, soft tissue, subcutaneous, bone, or multiple sites) were discovered by the patients, one chest wall recurrence was detected on physical examination, and one pelvic and one mediastinal recurrence was noted on MRI and CT scans, respectively. In two of the patients, routine CXR was suggestive of lung metastases and further staging revealed additional non-pulmonary metastases. Median time from last routine follow-up to relapse with non-pulmonary metastases was 3 (range 1–9) months (Figure 3).
Outcomes of relapsed patients
Local relapse
Fifteen of the 26 patients (58%) who experienced an isolated local recurrence are alive, and 12 of those patients (46%) are disease free with a median follow-up time of 22 (range 4–57) months after the last recurrence. Nine patients are deceased, and two are lost to follow-up.
In 20 of 26 patients, curative treatment for local tumour recurrence was undertaken: surgery alone in 10 patients, surgery and radiotherapy in 8 patients, surgery and chemotherapy in a patient with synovial sarcoma, and chemotherapy alone in a patient with extraosseus Ewing sarcoma. Eight patients did not have a further relapse. However, 12 patients had another recurrence: five were local (four of which were successfully salvaged — 2 with further local resections and two with subsequent limb amputation), four developed lung metastases, two developed local relapse with synchronous lung metastases, and one patient relapsed with non-pulmonary metastases (Figure 4).
Lung metastases
Nine of the 22 (41%) patients with imaging-detected asymptomatic lung metastases (seven on CXR, two on CT scan) underwent pulmonary metastatectomy with curative intent. Eight of these patients are alive and seven are currently disease free with a median follow-up time of 14 (range 6–38) months. In these seven patients, the interval between primary sarcoma treatment and detection of lung metastases was more than 6 months. Patients had one to four metastases on preoperative scanning, and a maximum of four metastases were removed at surgery. One patient developed lung metastases shortly after initial primary treatment. Whereas on the preoperative CT scan only 3 metastases were visible, at surgery more than 20 metastases were resected. This patient subsequently relapsed rapidly and died. Two patients were treated with radiofrequency ablation for recurrent lung metastases; one remains disease-free, while the other patient has relapsed again.
Thirteen patients with CXR- or CT-scan-detected lung metastases were considered unsuitable for pulmonary metastatectomy owing to the extent of disease, and were treated with palliative intent. Twelve patients have died since, and one patient was lost to follow-up and has presumably died. Median time from relapse to death was 13 (range 4–39) months.
The six patients with symptomatic lung relapse all had advanced disease without curative treatment options. Five patients died of metastatic sarcoma and one was lost to follow-up. The short interval from end of treatment to relapse indicates aggressive disease with rapid progression.
In summary, 41% of patients with lung metastases detected on routine imaging were treated with curative intent and 32% remain free of disease at present. Of note, the seven patients who underwent curative metastectomy and are disease free had a longer relapse-free interval from primary treatment of 30 (range 9–66) months compared with patients who had a subsequent relapse or were not deemed suitable for surgical intervention with a median relapse-free interval of 3 (range 1–22) months.
Non-pulmonary metastases
Thirteen of the 23 patients with non-pulmonary metastases have died from metastatic sarcoma. Median time from relapse to death was 8 (range 1–33) months. Two patients were lost to follow-up and nine patients are still alive, two of whom are currently disease free. Both of them had soft tissue metastases amenable to resection at relapse.
Discussion
The aim of this study was to assess the mode and effectiveness of detection of relapse in patients undergoing surveillance for extremity STS, and to identify optimal follow-up schedules. We retrospectively analysed data from 174 patients who were treated at a single centre over a period of 6 years. In our patient group with mostly high-grade and therefore high-risk STS, the rate of local relapse of 18% is consistent with other data sets in extremity STS (Lawrence et al, 1987; Lewis et al, 1997; Eilber et al, 2003), as is the rate of metastatic spread of 32% (Pisters et al, 1996; Huth and Eilber, 1988; Billingsley et al, 1999b). The aim of any surveillance programme is to identify relapses at such a time that the relapses can be successfully treated, achieving long-term survival. In our series, of the 31 patients with local recurrence, 46% are disease free at a median follow-up of 22 months after recurrence, and of the 28 patients with lung metastases 32% who underwent metastasectomy are disease free at a median follow-up of 14 months.
Of the 31 patients in our series with a local relapse, 97% of relapses were detected clinically, 22 by the patients themselves and 8 by physicians in clinic. Only one local recurrence was asymptomatic and detected on an MRI scan. This is consistent with a study that evaluated the role of routine surveillance MRI scanning in the detection of local recurrence for limb STS (Labarre et al, 2009). One hundred and twenty-four patients underwent 663 planned MRI scans according to a defined follow-up protocol. Of 11 local recurrences (9%), nine were detected clinically and only two were asymptomatic MRI-detected recurrences. This low screen detection rate came at a cost of 11 false-positive MRI scans. Overall, the authors concluded that clinical examination was a more effective method of surveillance compared with routine MRI scanning.
A proportion of patients with STS who develop isolated relapse in the lungs can be potentially cured by resection of lung metastases (Billingsley et al, 1999a). In a series of 719 STS patients with lung metastases, the median interval between the treatment of the primary tumour and the development of metastatic disease was 10 months. Relapse-free interval was a prognostic indicator, with an interval of 1 year or less being associated with poorer prognosis. In another series, the number of nodules detected by CT scanning preoperatively was found to have significant prognostic value, with poorer outcomes for patients with four or more nodules (Casson et al, 1992). Our patients with lung metastases who underwent subsequent resection and are disease free had a median relapse-free interval from primary treatment of 30 months, and had up to four nodules removed. Patients in our series with symptomatic lung metastases had a median time to relapse of 5 months, and none of these patients were suitable for surgery. It seems unlikely that more frequent surveillance would have altered outcomes in this patient group, which probably reflects aggressive tumour biology.
The challenge of early detection of local relapse and metastatic spread with a view to curative salvage treatment while preventing unnecessary and even harmful investigations is yet to be solved. Follow-up recommendations differ substantially in modality and intensity and routine practice is even more diverse (Sakata et al, 2003; Gerrand et al, 2007). In a small retrospective analysis of children diagnosed with relapsed soft tissue and bone sarcomas, regular imaging studies (chest CT, MRI, and PET) did not lead to earlier recognition of relapse and did not influence overall survival (Postovsky et al, 2008).
Previously, Whooley et al published data on the efficacy of their surveillance strategy in 174 patients with primary extremity STS and provided a cost-effectiveness analysis (Whooley et al, 1999, 2000). Importantly, only 49% of sarcomas were high grade compared with 90% in our series. They concluded differently in their two publications: whereas they advocated physical examination, clinical assessment of patient symptoms, and CXR imaging as effective strategies for follow-up of primary extremity STS in the first publication (Whooley et al, 1999), they subsequently postulated that more aggressive observation during the first 2 years after treatment with a 3-monthly physical examination and CXR and cross-sectional imaging every 6 months where indicated for 2 years would be appropriate in high-risk tumours with high grade, large size, and deep localisation in the second publication (Whooley et al, 2000). However, the accompanying editorial (Brennan, 2000) raised several appropriate still unanswered questions: ‘Is routine physician-directed follow-up justified if 50% of relapses are detected between regular visits? Is any imaging of the primary tumour required if 86% of local relapses were resectable and 19 of 20 were noticed on physical examination? Furthermore, taking into account the negative impact on outcome for a short interval from initial sarcoma treatment to pulmonary relapse, this could be an argument against frequent scanning during the first year of follow-up.’
The benefit and cost-effectiveness of chest CT as compared with CXR for staging at initial sarcoma diagnosis was evaluated in two studies (Fleming et al, 2001; Porter et al, 2002). However, these results cannot be extrapolated to the follow-up situation and do not inform on the best imaging modality. Recently a retrospective analysis of 176 patients who were followed up with CT scans or plain CXR for pulmonary metastasis monitoring after surgery for STS was reported (Cho et al, 2011). As expected, there was a trend towards unilaterality of lung metastases, smaller size, and management with metastasectomy in the CT cohort. The 2- and 4-year survival rates after detection of pulmonary metastasis were 20% and 0% in the plain radiograph cohort and 47% and 32% in the chest CT cohort, respectively. These results should be interpreted with caution because of the small number of patients in each cohort (26 and 28, respectively). In our series of patients with lung relapses that were mostly detected on CXR, outcomes appear broadly comparable to other reports (Billingsley et al, 1999a), although our patient numbers are small and follow-up is short for some patients.
There has been only one randomised trial of follow-up strategies in sarcoma patients (Puri et al, 2014). This compared modality of chest imaging (CXR and CT scans) and frequency of follow-up (3-monthly and 6-monthly visits) in 500 patients with extremity sarcomas (359 bone and 151 soft tissue sarcomas) in a two-by-two factorial design. The results demonstrated non-inferiority of CXR as compared with CT scans (3-year OS 67% and 66%, respectively; DFS 54% and 49%, respectively). Three year OS was similar for 3-monthy and 6-monthly visits (69% and 64%, respectively); yet the trial could not conclusively demonstrate non-inferiority for less frequent follow-up visits. Interestingly, almost 90% of local recurrences were found by patients themselves, which is very consistent with our findings.
ESMO guidelines for sarcoma acknowledge the lack of published data on the optimal routine follow-up of patients with localised disease treated by surgery (ESMO/European Sarcoma Network Working Group, 2012). Imaging of the lungs is advocated, in addition to clinical assessment of the primary site. Follow-up intervals of 3–4 months in the first 2–3 years, 6-monthly up to the 5th year, and annually thereafter are suggested.
Overall, regular CXR seems to be a feasible method of chest surveillance considering the positive and negative predictive values of surveillance CXR in STS of 92% and 97%, respectively (Whooley et al, 2000). In a review on surveillance strategies for patients following surgical resection of STS, the additional benefit of CT scans of the lung was considered low, and the importance of thorough history and physical examination was emphasised (Kane, 2004).
There are several limitations to our analysis: it was performed retrospectively and follow-up was not performed according to defined guidelines before April 2007. However, on comparing patients who underwent treatment during the years 2003–2006 and 2007–2009, the recurrence-free survival curves appeared similar and no significant changes in the relapse pattern could be observed (P=0.76, log-rank test). We only included STS of the limb in this study and therefore no conclusions for surveillance strategies in STS of other locations can be drawn, especially for retroperitoneal sarcomas, where clinical assessment is difficult. Another limitation of our study is in the heterogeneity of STS and the diverse biological behaviour of the >50 histological subtypes, which makes generalisations difficult. Furthermore, we are unable to present an optimal schedule for the follow-up of soft tissue sarcomas of the limb. However, we can conclude that regular physical examination of the primary tumour site detects the vast majority of local relapses. Moreover, in the light of our results and the recent prospective randomised trial comparing radiological modalities in the follow-up of extremity sarcomas (Puri et al, 2014), it appears that CXR may well be sufficient to detect lung metastases amenable to resection, and that regular CT scans of the lungs are unlikely to yield better results.
Conclusion
Patients with recurrent STS of the limb can potentially be salvaged either by resection of local recurrence or by resection of distant (usually lung) metastases. Surveillance should be directed at detection of local relapse or lung metastases. Patient education and awareness to detect local relapse should be encouraged. As a minimum, regular CXR to detect lung metastases amenable to resection should be performed, although the optimal interval of imaging is not known, and it seems that patients developing lung metastases during the first year after primary treatment have particularly poor outcomes. The definition of a rational evidence-based follow-up programme based on the risk of recurrence remains to be established and needs prospective evaluation in clinical trials.
Change history
13 May 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Brookmeyer R, Crowley J (1982) A confidence interval for the median survival time. Biometrics 38 (1): 29–41.
Ballo MT, Zagars GK, Cormier JN, Hunt KK, Feig BW, Patel SR, Pisters PW (2004) Interval between surgery and radiotherapy: effect on local control of soft tissue sarcoma. Int J Radiat Oncol Biol Phys 58 (5): 1461–1467.
Barkley HT Jr ., Martin RG, Romsdahl MM, Lindberg R, Zagars GK (1988) Treatment of soft tissue sarcomas by preoperative irradiation and conservative surgical resection. Int J Radiat Oncol Biol Phys 14 (4): 693–699.
Billingsley KG, Burt ME, Jara E, Ginsberg RJ, Woodruff JM, Leung DH, Brennan MF (1999a) Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann Surg 229 (5): 602–610, (discussion 610–2).
Billingsley KG, Lewis JJ, Leung DH, Casper ES, Woodruff JM, Brennan MF (1999b) Multifactorial analysis of the survival of patients with distant metastasis arising from primary extremity sarcoma. Cancer 85 (2): 389–395.
Brennan MF (2000) Follow-up is valuable and effective: true, true and unrelated? Ann Surg oncol 7 (1): 2–3.
Brenner DJ, Hall EJ (2007) Computed tomography–an increasing source of radiation exposure. New Engl J Med 357 (22): 2277–2284.
Casson AG, Putnam JB, Natarajan G, Johnston DA, Mountain C, McMurtrey M, Roth JA (1992) Five-year survival after pulmonary metastasectomy for adult soft tissue sarcoma. Cancer 69 (3): 662–668.
Cho HS, Park IH, Jeong WJ, Han I, Kim HS (2011) Prognostic value of computed tomography for monitoring pulmonary metastases in soft tissue sarcoma patients after surgical management: a retrospective cohort study. Ann Surg Oncol 18 (12): 3392–3398.
Coindre JM, Nguyen BB, Bonichon F, de Mascarel I, Trojani M (1988) Histopathologic grading in spindle cell soft tissue sarcomas. Cancer 61 (11): 2305–2309.
CORE TEAM R (2013) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria.
Eilber FC, Rosen G, Nelson SD, Selch M, Dorey F, Eckardt J, Eilber FR (2003) High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg 237 (2): 218–226.
ESMO/European Sarcoma Network Working Group (2012) Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 23 (Suppl 7): vii92–vii99.
Figueredo A, Rumble RB, Maroun J, Earle CC, Cummings B, McLeod R, Zuraw L, Zwaal C (2003) Follow-up of patients with curatively resected colorectal cancer: a practice guideline. BMC Cancer 3: 26.
Fleming JB, Cantor SB, Varma DG, Holst D, Feig BW, Hunt KK, Patel SR, Benjamin RS, Pollock RE, Pisters PW (2001) Utility of chest computed tomography for staging in patients with T1 extremity soft tissue sarcomas. Cancer 92 (4): 863–868.
Gerrand CH, Billingham LJ, Woll PJ, Grimer RJ (2007) Follow up after primary treatment of soft tissue sarcoma: a survey of current practice in the United Kingdom. Sarcoma 2007: 34128.
Grimer R, Judson I, Peake D, Seddon B (2010) Guidelines for the management of soft tissue sarcomas. Sarcoma 2010: 506182.
Haglund KE, Raut CP, Nascimento AF, Wang Q, George S, Baldini EH (2012) Recurrence patterns and survival for patients with intermediate- and high-grade myxofibrosarcoma. Int J Radiat Oncol Biol Phy 82 (1): 361–367.
Huth JF, Eilber FR (1988) Patterns of metastatic spread following resection of extremity soft-tissue sarcomas and strategies for treatment. Semin Surg Oncol 4 (1): 20–26.
Kane JM 3rd (2004) Surveillance strategies for patients following surgical resection of soft tissue sarcomas. Curr Opin Oncol 16 (4): 328–332.
Kaplan EL (1958) MP Nonparametric estimations for incomplete observations. J Am Stat Assoc 53: 457–481.
Krieg AH, Hefti F, Speth BM, Jundt G, Guillou L, Exner UG, von Hochstetter AR, Cserhati MD, Fuchs B, Mouhsine E, Kaelin A, Klenke FM, Siebenrock KA (2011) Synovial sarcomas usually metastasize after >5 years: a multicenter retrospective analysis with minimum follow-up of 10 years for survivors. Ann Oncol 22 (2): 458–467.
Labarre D, Aziza R, Filleron T, Delannes M, Delaunay F, Marques B, Ferron G, Chevreau C (2009) Detection of local recurrences of limb soft tissue sarcomas: is magnetic resonance imaging (MRI) relevant? Eur J Radiol 72 (1): 50–53.
Lawrence W Jr ., Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D (1987) Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg 205 (4): 349–359.
Lewis JJ, Leung D, Heslin M, Woodruff JM, Brennan MF (1997) Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol 15 (2): 646–652.
National Cancer Intelligence Network (2012) Soft tissue sarcoma incidence and survival tumours diagnosed in England between 1985 and 2009. http://www.ncin.org.uk/cancer_type_and_topic_specific_work/cancer_type_specific_work/sarcomas/ (accessed 11 January 2014).
NCCN (2014) http://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf.
O'Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Kandel R, Goddard K, Sadura A, Pater J, Zee B (2002) Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359 (9325): 2235–2241.
Palli D, Russo A, Saieva C, Ciatto S, Rosselli Del Turco M, Distante V, Pacini P (1999) Intensive vs clinical follow-up after treatment of primary breast cancer: 10-year update of a randomized trial. National Research Council Project on Breast Cancer Follow-up. JAMA 281 (17): 1586.
Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF (1996) Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 14 (5): 1679–1689.
Porter GA, Cantor SB, Ahmad SA, Lenert JT, Ballo MT, Hunt KK, Feig BW, Patel SR, Benjamin RS, Pollock RE, Pisters PW (2002) Cost-effectiveness of staging computed tomography of the chest in patients with T2 soft tissue sarcomas. Cancer 94 (1): 197–204.
Postovsky S, Barzilai M, Meller I, Kollander Y, Futerman B, Ben Arush MW (2008) Does regular follow-up influence the survival of patients with sarcoma after recurrence? The Miri Shitrit pediatric oncology department experience. J Pediat HematolOncol 30 (3): 189–195.
Potter DA, Kinsella T, Glatstein E, Wesley R, White DE, Seipp CA, Chang AE, Lack EE, Costa J, Rosenberg SA (1986) High-grade soft tissue sarcomas of the extremities. Cancer 58 (1): 190–205.
Puri A, Gulia A, Hawaldar R, Ranganathan P, Badwe RA (2014) Does intensity of surveillance affect survival after surgery for sarcomas? Results of a randomized noninferiority trial. Clin Orthop Relat Res 472 (5): 1568–1575.
Rosenberg SA, Tepper J, Glatstein E, Costa J, Baker A, Brennan M, DeMoss EV, Seipp C, Sindelar WF, Sugarbaker P, Wesley R (1982) The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 196 (3): 305–315.
Rustin GJ, Mead GM, Stenning SP, Vasey PA, Aass N, Huddart RA, Sokal MP, Joffe JK, Harland SJ, Kirk SJ (2007) Randomized trial of two or five computed tomography scans in the surveillance of patients with stage I nonseminomatous germ cell tumors of the testis: Medical Research Council Trial TE08, ISRCTN56475197–the National Cancer Research Institute Testis Cancer Clinical Studies Group. J Clin Oncol 25 (11): 1310–1315.
Rustin GJ, van der Burg ME, Griffin CL, Guthrie D, Lamont A, Jayson GC, Kristensen G, Mediola C, Coens C, Qian W, Parmar MK, Swart AM (2010) Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet 376 (9747): 1155–1163.
Sakata K, Johnson FE, Beitler AL, Kraybill WG, Virgo KS (2003) Extremity soft tissue sarcoma patient follow-up: tumor grade and size affect surveillance strategies after potentially curative surgery. Int J Oncol 22 (6): 1335–1343.
Tarin TV, Sonn G, Shinghal R (2009) Estimating the risk of cancer associated with imaging related radiation during surveillance for stage I testicular cancer using computerized tomography. J Urol 181 (2): 627–632, discussion 632–633.
Thompson CA, Charlson ME, Schenkein E, Wells MT, Furman RR, Elstrom R, Ruan J, Martin P, Leonard JP (2010) Surveillance CT scans are a source of anxiety and fear of recurrence in long-term lymphoma survivors. Ann Oncol 21 (11): 2262–2266.
Thompson CA, Maurer MJ, Ghesquieres H, Macon WR, Habermann TM, Witzig TE, Cerhan JR, Link BK (2013) Utility of post-therapy surveillance scans in DLBCL. J Clin Oncol 31 (Suppl): abstr 8504.
Wanebo HJ, Temple WJ, Popp MB, Constable W, Aron B, Cunningham SL (1995) Preoperative regional therapy for extremity sarcoma. A tricenter update. Cancer 75 (9): 2299–2306.
Whooley BP, Gibbs JF, Mooney MM, McGrath BE, Kraybill WG (2000) Primary extremity sarcoma: what is the appropriate follow-up? Ann Surg Oncol 7 (1): 9–14.
Whooley BP, Mooney MM, Gibbs JF, Kraybill WG (1999) Effective follow-up strategies in soft tissue sarcoma. Sem SurgOncol 17 (1): 83–87.
Wilson AN, Davis A, Bell RS, O'Sullivan B, Catton C, Madadi F, Kandel R, Fornasier VL (1994) Local control of soft tissue sarcoma of the extremity: the experience of a multidisciplinary sarcoma group with definitive surgery and radiotherapy. Eur J Cancer 30A (6): 746–751.
Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, DeLaney T, Glatstein E, Steinberg SM, Merino MJ, Rosenberg SA (1998) Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 16 (1): 197–203.
Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS, Evans HL (2003) Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer 97 (10): 2530–2543.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Rothermundt, C., Whelan, J., Dileo, P. et al. What is the role of routine follow-up for localised limb soft tissue sarcomas? A retrospective analysis of 174 patients. Br J Cancer 110, 2420–2426 (2014). https://doi.org/10.1038/bjc.2014.200
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2014.200
Keywords
This article is cited by
-
Enhancing local recurrence detection in patients with high-grade soft tissue sarcoma: value of short-term Ultrasonography added to post-operative MRI surveillance
Cancer Imaging (2024)
-
The value of re-staging chest CT at first local recurrence of extremity and trunk soft tissue sarcoma
European Radiology (2021)
-
Cost-effectiveness analysis of imaging surveillance in stage II and III extremity soft tissue sarcoma: an Australian perspective
Cost Effectiveness and Resource Allocation (2020)
-
Can we use MRI to detect clinically silent recurrent soft-tissue sarcoma?
European Radiology (2020)
-
Weichteiltumorresektionen
Der Orthopäde (2020)