Abstract
Background:
The value of a combined index of neutrophil and white cell counts, named derived neutrophil–lymphocyte ratio (dNLR), has recently been proposed as a prognosticator of survival in various cancer types. We investigated the prognostic role of the dNLR in a large European cohort of patients with upper tract urothelial carcinoma (UTUC).
Methods:
Data from 171 non-metastatic UTUC patients, operated between 1990 and 2012 at a single tertiary academic centre, were evaluated retrospectively. Cancer-specific- (CSS) as well as overall survival (OS) were assessed using the Kaplan–Meier method. To evaluate the independent prognostic significance of the dNLR, multivariate proportional Cox-regression models were applied. Additionally, the influence of the dNLR on the predictive accuracy of the multivariate model was further determined by Harrell’s concordance index (c-index).
Results:
The median follow-up period was 31 months. An increased dNLR was statistically significantly associated with shorter CSS (log-rank P=0.004), as well as with shorter OS (log-rank P=0.002). Multivariate analysis identified dNLR as an independent predictor for CSS (hazard ratio, HR=1.16, 95% confidence interval, CI=1.01–1.35, P=0.045), as well as for OS (HR=1.21, 95% CI=1.09–1.34, P<0.001). The estimated c-index of the multivariate model for OS was 0.68 without dNLR and 0.73 when dNLR was added.
Conclusions:
Patients with a high pretreatment dNLR could be predicted to show subsequently higher cancer-specific- as well as overall mortality after surgery for UTUC compared with those with a low pretreatment dNLR. Thus, this combined index should be considered as a potential prognostic biomarker in future.
Similar content being viewed by others
Main
Upper tract urothelial carcinoma (UTUC) represents a relatively rare, albeit in most cases highly malignant, disease accounting for ∼5–10% of all urothelial carcinomas (Rouprêt et al, 2011). At present, the estimated annual incidence rate of UTUC in western countries is about 2/100.000 inhabitants (Jemal et al, 2009; Rouprêt et al, 2011). Up to 60% of UTUCs present invasive at the time of diagnosis compared with only 15–25% of malignant bladder tumours. UTUCs invading the muscle wall are in general considered to represent highly aggressive tumours with an accordingly poor prognosis (Rouprêt et al, 2011). Five-year cancer-specific survival (CSS) rates are <50% for locally muscle-invasive tumours and even <10% for locally advanced ones (Fajkovic et al, 2013). According to the recent literature, prognostic factors of paramount importance in UTUC are pathologic (pT) tumour stage and grade (Drouin et al, 2013; Rouprêt et al, 2013). Other prognostic factors that were shown to have a significant role in UTUC include lymphovascular invasion and histologic tumour necrosis (Zigeuner et al, 2010). In contrast, the knowledge of preoperatively assessable prognostic factors in UTUC is still limited. Moreover, none of blood- or tissue-based potential biomarkers have fulfilled the clinical and statistical criteria necessary to support their introduction into routine clinical practice in UTUC so far.
There is a growing body of evidence supporting the role of the immune response as an important factor in human cancer development and progression (Clarke et al, 2011). Several markers of systemic inflammatory response, such as the pretreatment plasma fibrinogen level, C-reactive protein, neutrophil- or platelet counts, as well as the neutrophil–lymphocyte ratio (NLR), have been shown to represent independent prognostic factors in various human cancer types (Clarke et al, 2011; Gondo et al, 2012; Hashimoto et al, 2013; Perez et al, 2013; Pichler et al, 2013a, 2013b; Stotz et al, 2013; Szkandera et al, 2013a; Tanaka et al, 2014). In particular, the pretreatment NLR might be regarded as an easily measureable and reproducible marker of systemic immune response, having a potential role in renal cell carcinoma as well as in UTUC (Pichler et al, 2013a). In a recently published report, we were able to confirm the role of pretreatment NLR as an independent prognosticator in UTUC patients (Dalpiaz et al, 2013). Regarding routinely measured laboratory parameters, in general only white cell and neutrophil counts, are commonly entered into clinical trial databases; therefore, in an attempt to obviate this problem, Proctor et al (2012) recently implemented a derived score, named the derived neutrophil–lymphocyte ratio (dNLR), which is composed of neutrophil count to (white cell count−neutrophil count). They evaluated the potential prognostic value of the dNLR on cancer outcomes in various human cancer types and were able to demonstrate that the dNLR had similar prognostic value as the established NLR (Proctor et al, 2012). More recently, other studies confirmed an independent prognostic value of the dNLR in certain types of cancer; however, they proposed different optimal cutoff values in different types of cancer (Absenger et al, 2013; Szkandera et al, 2013b). Thus, we decided to validate the prognostic significance of the pretreatment dNLR in a large European cohort of patients with non-metastatic UTUC with regard to patients’ CSS as well as overall survival (OS).
Materials and Methods
This retrospective analysis included data from 171 patients of a cohort of 202 consecutive non-metastatic UTUC patients who underwent radical nephroureterectomy or segmental ureterectomy at the Department of Urology at the Medical University of Graz, between September 1990 and July 2012. Thirty-one (15.3%) patients of the whole cohort (n=202) could not be included into analyses due to at least one missing laboratory parameter at the time of surgical intervention. To analyse whether there are any differences between the excluded (n=31) patients and the actually analysed cases (n=171), we compared all available clinicopathological parameters between these two groups. All clinicopathological data were retrieved from medical records at the Department of Urology, as well as from pathology reports at the Institute of Pathology at the same institution. Segmental ureterectomy was conducted in imperative (solitary kidney, chronic renal insufficiency, impaired renal function or parenchymal rarification of the contralateral kidney or American Society of Anesthesiologists score 4) or in elective cases, if the lesion was unifocally located in the distal ureter. Pathologic T-stages were uniformly adjusted according to the TNM 2009 classification system; tumour grade was assessed according to the WHO 1973 guidelines (Lopez-Beltran et al, 2004; Sobin et al, 2009). Additionally, data on tumour site and location, presence or absence (not quantitatively assessed) of histologic tumour necrosis, surgical resection margins, as well as patients’ age and gender were retrieved from patients’ medical/pathological records as mentioned above. All laboratory data, including neutrophil and lymphocyte counts, were obtained during patients’ hospitalisation before surgery. The dNLR was calculated according to the original publication by Proctor et al (2012) as follows: dNLR=neutrophil count to (white cell count−neutrophil count). Patients’ follow-up included a physical examination, cystoscopy and urinary cytology, as well as radiological assessment (CT or MRI) for at least 5 years, according to the current (2013) European Association of Urology guidelines (Rouprêt et al, 2011). No neoadjuvant treatment was administered. Patients’ time of death was obtained from the central registry of the Austrian Bureau of Statistics. Cancer-related death was coded as a cancer-specific event. All other deaths were considered as other-cause mortality. The median time of follow-up was calculated using the time to patients’ last follow-up or death.
Statistical analyses
Primary study end points consisted of patients’ OS and CSS. Receiver operating characteristic (ROC) curve analysis was used to determine differences between the NLR and dNLR. The optimal cutoff values for the dNLR were determined by ROC analysis. The dNLR was subsequently correlated with clinicopathological features by the χ2 test. The association between the clinicopathological features and the dNLR with patients’ CSS and OS was analysed using Kaplan–Meier curves and compared by the log-rank test. In the multivariate Cox-regression analysis, the model was adjusted for prognostic clinicopathological factors that were statistically significantly associated with CSS and OS in univariate analyses. Hazard ratios (HRs) estimated from the Cox analysis are reported as relative risks with corresponding 95% confidence intervals (CIs). Harrell’s concordance index (c-index) was used for the assessment of the predictive accuracy of the model in multivariable analyses, as well as to compare after supplementation by the dNLR (Pichler et al, 2011).
All statistical analyses were performed using the Statistical Package for Social Sciences version 20.0 (SPSS Inc., Chicago, IL, USA), MedCalc or STATA software. A two-sided P<0.05 was considered statistically significant.
Results
Overall, there were 107 (62.6%) male and 64 (37.4%) female subjects in the study cohort. The mean age of the study cohort at the time of surgery was 69±10.1 years. In 95 (55.6%) patients, the tumour was located in the renal pelvis and in 76 (44.4%) patients the tumour was located in the ureter. At the pathological examination, 79 (46.2%) patients showed a pT1 tumour and 92 (53.8%) patients had a muscle-invasive tumour or advanced disease state (pT2–4). Tumour grades were distributed as follow: G1–2 in 92 (53.8%) and G3–4 in 79 (46.2%) patients. Histologic tumour necrosis was noted in 21 (12.3%) patients. There were no significant differences in the distribution of clinicopathological factors between the 171 analysed cases and the 31 excluded cases (Supplementary Table S1). The mean dNLR was 2.6±1.8. Seventy-nine (46.2%) patients died within the study period, of which 54 (31.6%) had a cancer-related death. The ROC curves, using CSS and OS as an end point for NLR and dNLR, are shown in Supplementary Figures 1 and 2. The ROC curves for NLR and dNLR were 0.603 (0.525–0.677) and 0.586 (0.508–0.660) for CSS (P=0.506) and 0.649 (0.572–0.720) and 0.629 (0.552–0.702) for OS (P=0.434), respectively. The statistically significant correlation between the NLR and dNLR was 0.833 (P<0.001, Spearman correlation). Additionally, we analysed the relationship of the NLR and dNLR to other potential prognostic clinicopathological factors. Only for the dNLR, a statistically significant association (P=0.044) with higher tumour grade could be observed (Table 1). The median follow-up was 31 months (interquartile range 13–69 months).
To investigate whether the dNLR is associated with patients’ clinical outcomes, univariate and multivariate analyses were performed. Univariate analysis identified high pT-stage (pT-2–4, P<0.001), high tumour grade (G1–2 vs G3–4, P<0.001), the presence of histologic tumour necrosis (P<0.001), as well as an increased dNLR (continuous variable P=0.024) as prognosticators of poor CSS, whereas gender, older (⩾65 yrs.) age, period of surgery, tumour site and tumour location were not associated with patients’ CSS in a statistically significant manner (Table 2).
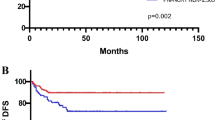
Figures 1 and 2 show the Kaplan–Meier curves for patients’ OS, as well as for CSS, and reveal that an elevated (an optimal cutoff value was calculated by ROC analysis of ⩾1.5) dNLR represented a strong and robust factor for decreased survival rates at 10 years in UTUC patients in the cohort studied (log-rank P<0.004 and <0.002). To determine the independent prognostic significance of the dNLR for patients’ CSS, a multivariate analysis using a Cox proportional hazard model was performed. The model revealed that pT-stage (HR=2.34, 95% CI=1.14–4.80, P=0.021), tumour grade (HR=2.05, 95% CI=1.09–3.83, P=0.025), the presence of histologic tumour necrosis (HR=2.16, 95% CI=1.09–4.29, P=0.028), as well as the (continuously coded) dNLR (HR=1.16, 95% CI=1.01–1.35, P=0.045) were independent predictors of CSS (Table 2). Regarding OS, high (⩾65yrs.) age at the time of surgery (HR=2.02, 95% CI=1.13–3.60, P=0.017), muscle-invasive pT-stage (HR=1.97, 95% CI=1.12–3.44, P=0.018), as well as an elevated (⩾1.5) dNLR (HR=1.21, 95% CI=1.09–1.34, P<0.001) were confirmed as independent prognosticators (Table 2). Harrell’s c-index of the multivariate model for OS was 0.68 and 0.73 when dNLR was supplemented, whereas Harrell’s c-index for CSS was 0.74 without and 0.75 with the dNLR supplemented.
Discussion
The results of the present study clearly indicate that the pretreatment dNLR might be considered as a significant prognostic factor in UTUC patients, allowing to potentially better predicted survival after surgical treatment. The hypothesis that the inflammatory response might be heavily involved in the natural history of various human cancer types has been confirmed by several studies, as mentioned before, and as both the NLR and the dNLR have already been shown to be able to predict survival in different cancer types (Proctor et al, 2012; Absenger et al, 2013; Dalpiaz et al, 2013; Shibutani et al, 2013; Stotz et al, 2013). The combined index dNLR is derived from the assumption that the white cell count is made up primarily of lymphocytes and neutrophils, and, therefore, the white cell count minus the neutrophil count would be broadly similar to lymphocyte counts. Proctor et al (2012) were the first to test the potential prognostic significance of the dNLR compared with the NLR alone in more than 12 000 patients with different cancer types. Both measures showed a similar prognostic value in a large cohort of unselected cancer patients. The authors hypothesised that the dNLR is broadly mixing two cell types, namely lymphocytes and monocytes, with possible opposing effects in terms of predictive value. In the normal range, the relative proportion of lymphocytes to monocytes is regarded to be ∼6 : 1. Even if there might be a fall in the absolute proportion of lymphocytes and an increase in the absolute proportion of monocytes in cancer patients, the white blood count minus monocytes is dominated by lymphocytes. Therefore, it seems highly likely that the dNLR represents a reasonable approximation of the NLR, and the potential error introduced by the presence of monocytes in the fraction is therefore likely to be small (Proctor et al, 2012).
In a recent study, Absenger et al (2013) tested the effect of preoperatively assessed dNLR in patients with stages II and III colon cancer. In their study cohort, the dNLR represented an independent prognostic marker for patients’ OS and time to recurrence in patients with advanced colon cancer.
The potential prognostic role of the NLR in urological cancer types has so far been confirmed only in a few studies (Gondo et al, 2012; Dalpiaz et al, 2013; Pichler et al, 2013a). In our study, we confirmed that patients with a high preoperative NLR had a subsequently higher cancer-specific- as well as overall mortality after radical surgery for UTUC compared with those with a low preoperative NLR (Dalpiaz et al, 2013). With the same aims we tested the prognostic role of the dNLR after radical surgery for UTUC. Our recent study clearly indicates that a high dNLR might be an independent predictor of survival in UTUC patients. To the best of our knowledge, this is the first study to evaluate the potential prognostic impact of pretreatment dNLR in UTUC patients with regard to CSS and OS. The ideal cutoff value in our study for dNLR was 1.5. Therefore, our results should be interpreted with caution, as the ideal threshold for the continuously coded dNLR was calculated by testing all possible thresholds that would discriminate between patients’ survival and cancer-related death by Cox proportional analyses. Furthermore, an ideal and generalisable dNLR threshold in UTUC has yet to be determined. In the present study, pathologic T-stage, tumour grade, the presence of histologic tumour necrosis and the (continuously coded) dNLR represented independent predictors of patients’ CSS. It has to be emphasised that currently pathologic T-stage represents the most important prognostic factor in UTUC in the largest published series (Novara et al, 2007; Jeldres et al, 2010). In the present study, we support the role of preoperatively available inflammatory parameters as useful potential biomarkers in UTUC, particularly because of low associated costs and easy accessibility. Nevertheless, our results should be interpreted with caution, as several potentially confounding factors are related to patients’ inflammatory responses, comorbidities, as well as to known study limitations such as retrospective design and data evaluation. A future validation of our findings in prospective multicentre series is warranted.
Change history
13 May 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Absenger G, Szkandera J, Pichler M, Stotz M, Arminger F, Weissmueller M, Schaberl-Moser R, Samonigg H, Stojakovic T, Gerger A (2013) A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br J Cancer 109 (2): 395–400.
Clarke SJ, Chua W, Moore M, Kao S, Phan V, Tan C, Charles K, McMillan DC (2011) Use of inflammatory markers to guide cancer treatment. Clin Pharmacol Ther 90 (3): 475–478.
Dalpiaz O, Ehrlich GC, Mannweiler S, Hernández JM, Gerger A, Stojakovic T, Pummer K, Zigeuner R, Pichler M, Hutterer GC (2013) Validation of the pre-treatment neutrophil-lymphocyte ratio as prognostic factor in a European cohort of patients with upper tract urothelial carcinoma. BJU Int e-pub ahead of print 5 September 2013 doi:10.1111/bju.12441.
Drouin SJ, Yates DR, Hupertan V, Cussenot O, Rouprêt M (2013) A systematic review of the tools available for predicting survival and managing patients with urothelial carcinomas of the bladder and of the upper tract in a curative setting. World J Urol 31 (1): 109–116.
Fajkovic H, Cha EK, Xylinas E, Rink M, Pycha A, Seitz C, Bolenz C, Dunning A, Novara G, Trinh QD, Karakiewicz PI, Margulis V, Raman JD, Walton TJ, Baba S, Carballido J, Otto W, Montorsi F, Lotan Y, Kassouf W, Fritsche HM, Bensalah K, Zigeuner R, Scherr DS, Sonpavde G, Roupret M, Shariat SF (2013) Disease-free survival as a surrogate for overall survival in upper tract urothelial carcinoma. World J Urol 31 (1): 5–11.
Gondo T, Nakashima J, Ohno Y, Choichiro O, Horiguchi Y, Namiki K, Yoshioka K, Ohori M, Hatano T, Tachibana M (2012) Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology 79 (5): 1085–1091.
Hashimoto T, Ohno Y, Nakashima J, Gondo T, Ohori M, Tachibana M (2013) Clinical significance of preoperative peripheral blood neutrophil count in patients with non-metastatic upper urinary tract carcinoma. World J Urol 31 (4): 953–958.
Jeldres C, Sun M, Lughezzani G, Isbarn H, Shariat SF, Widmer H, Graefen M, Montorsi F, Perrotte P, Karakiewicz PI (2010) Highly predictive survival nomogram after upper urinary tract urothelial carcinoma. Cancer 116 (16): 3774–3784.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59 (4): 225–249.
Lopez-Beltran A, Bassi P, Pavone-Macaluso M, Montironi R (2004) Handling and pathology reporting of specimens with carcinoma of the urinary bladder, ureter, and renal pelvis. Eur Urol 45 (3): 257–266.
Novara G, De Marco V, Gottardo F, Dalpiaz O, Bouygues V, Galfano A, Martignoni G, Patard JJ, Artibani W, Ficarra V (2007) Independent predictors of cancer-specific survival in transitional cell carcinoma of the upper urinary tract: multi-institutional dataset from 3 European centers. Cancer 110 (8): 1715–1722.
Perez DR, Baser RE, Cavnar MJ, Balachandran VP, Antonescu CR, Tap WD, Strong VE, Brennan MF, Coit DG, Singer S, Dematteo RP (2013) Blood neutrophil-to-lymphocyte ratio is prognostic in gastrointestinal stromal tumor. Ann Surg Oncol 20 (2): 593–599.
Pichler M, Hutterer GC, Chromecki TF, Jesche J, Kampel-Kettner K, Rehak P, Pummer K, Zigeuner R (2011) External validation of the Leibovich prognosis score for nonmetastatic clear cell renal cell carcinoma at a single European center applying routine pathology. J Urol 186 (5): 1773–1777.
Pichler M, Hutterer GC, Stoeckigt C, Chromecki TF, Stojakovic T, Golbeck S, Eberhard K, Gerger A, Mannweiler S, Pummer K, Zigeuner R (2013a) Validation of the pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in a large European cohort of renal cell carcinoma patients. Br J Cancer 108 (4): 901–907.
Pichler M, Hutterer GC, Stojakovic T, Mannweiler S, Pummer K, Zigeuner R (2013b) High plasma fibrinogen level represents an independent negative prognostic factor regarding cancer-specific, metastasis-free, as well as overall survival in a European cohort of non-metastatic renal cell carcinoma patients. Br J Cancer 109 (5): 1123–1129.
Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ (2012) A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer 107 (4): 695–699.
Rouprêt M, Zigeuner R, Palou J, Boehle A, Kaasinen E, Sylvester R, Babjuk M, Oosterlinck W (2011) European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol 59 (4): 584–594.
Rouprêt M, Hupertan V, Seisen T, Colin P, Xylinas E, Yates DR, Fajkovic H, Lotan Y, Raman JD, Zigeuner R, Remzi M, Bolenz C, Novara G, Kassouf W, Ouzzane A, Rozet F, Cussenot O, Martinez-Salamanca JI, Fritsche HM, Walton TJ, Wood CG, Bensalah K, Karakiewicz PI, Montorsi F, Margulis V, Shariat SF (2013) Prediction of cancer specific survival after radical nephroureterectomy for upper tract urothelial carcinoma: development of an optimized postoperative nomogram using decision curve analysis. J Urol 189 (5): 1662–1669.
Shibutani M, Maeda K, Nagahara H, Noda E, Ohtani H, Nishiguchi Y, Hirakawa K (2013) A high preoperative neutrophil-to-lymphocyte ratio is associated with poor survival in patients with colorectal cancer. Anticancer Res 33 (8): 3291–3294.
Sobin L, Gospodarowicz M, Wittekind C (2009) TNM Classification of Malignant Tumours. Urological Tumours. Renal Pelvis and Ureter 7 edn. Wiley-Blackwell, uicc, 2009, pp 258–261, . Available at http://www.uicc.org/tnm/.
Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner C, Stojakovic T, Samonigg H, Hoefler G, Pichler M (2013) Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer 109 (2): 416–421.
Szkandera J, Absenger G, Liegl-Atzwanger B, Pichler M, Stotz M, Samonigg H, Glehr M, Zacherl M, Stojakovic T, Gerger A, Leithner A (2013a) Elevated preoperative neutrophil/lymphocyte ratio is associated with poor prognosis in soft-tissue sarcoma patients. Br J Cancer 108 (8): 1677–1683.
Szkandera J, Stotz M, Eisner F, Absenger G, Stojakovic T, Samonigg H, Kornprat P, Schaberl-Moser R, Alzoughbi W, Ress AL, Seggewies FS, Gerger A, Hoefler G, Pichler M (2013b) External validation of the derived neutrophil to lymphocyte ratio as a prognostic marker on a large cohort of pancreatic cancer patients. PLoS One 8 (11): e78225.
Tanaka N, Kikuchi E, Shirotake S, Kanao K, Matsumoto K, Kobayashi H, Miyazaki Y, Ide H, Obata J, Hoshino K, Hayakawa N, Ito Y, Kosaka T, Kodaira K, Oyama M, Miyajima A, Momma T, Nakagawa K, Ueno M, Oya M (2014) The predictive value of C-reactive protein for prognosis in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy: A Multi-institutional Study. Eur Urol 65 (1): 227–234.
Zigeuner R, Shariat SF, Margulis V, Karakiewicz PI, Roscigno M, Weizer A, Kikuchi E, Remzi M, Raman JD, Bolenz C, Bensalah K, Capitanio U, Koppie TM, Kassouf W, Sircar K, Patard JJ, Fernández MI, Wood CG, Montorsi F, Ströbel P, Wheat JC, Haitel A, Oya M, Guo CC, Ng C, Chade DC, Sagalowsky A, Langner C (2010) Tumour necrosis is an indicator of aggressive biology in patients with urothelial carcinoma of the upper urinary tract. Eur Urol 57 (4): 575–581.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Dalpiaz, O., Pichler, M., Mannweiler, S. et al. Validation of the pretreatment derived neutrophil–lymphocyte ratio as a prognostic factor in a European cohort of patients with upper tract urothelial carcinoma. Br J Cancer 110, 2531–2536 (2014). https://doi.org/10.1038/bjc.2014.180
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2014.180
Keywords
This article is cited by
-
Preoperative fibrinogen/CRP score predicts survival in upper urothelial tract carcinoma patients undergoing radical curative surgery
World Journal of Urology (2023)
-
Red cell differential width (RDW) as a predictor of survival outcomes with palliative and adjuvant chemotherapy for metastatic penile cancer
International Urology and Nephrology (2020)
-
Critical evaluation of the potential prognostic value of the pretreatment-derived neutrophil–lymphocyte ratio under consideration of C-reactive protein levels in clear cell renal cell carcinoma
British Journal of Cancer (2017)
-
Prognostic value of preoperative NLR, dNLR, PLR and CRP in surgical renal cell carcinoma patients
World Journal of Urology (2017)
-
Promising role of preoperative neutrophil-to-lymphocyte ratio in patients treated with radical nephroureterectomy
World Journal of Urology (2017)