Abstract
Purpose. Phenethyl isothiocyanate (PEITC) has been of great interest as a promising cancer chemopreventive agent. To better understand its chemopreventive activity, we examined the effect of PEITC on the antioxidant responsive element (ARE), which is an important gene regulatory element of many phase II drug-metabolizing/detoxification enzymes as well as cellular defensive enzymes.
Methods. HeLa cells were transiently transfected with different cDNA plasmids using calcium phosphate precipitation. Subsequently, the cells were maintained in fresh media, and various concentrations of PEITC were added to the transfected cells. After harvesting and lysing of the cells, ARE-luciferase reporter gene activity was measured and normalized against β-galactosidase activity.
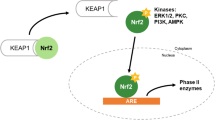
Results. Treatments of HeLa cells with PEITC transiently stimulated ARE-reporter gene expressions in a dose-dependent manner. Overexpression of wild-type NF-E2 related factor-2 (Nrf2) dramatically increased ARE-reporter gene expression in a dose-dependent manner. Similar effects were seen when wild-type c-Jun N-terminal kinase 1 (JNK1) was transfected, although the transactivating potential of JNK1 was much less than that of Nrf2. Cotransfection of Nrf2 and JNK1 showed additional enhancement of ARE reporter gene expression, implying that JNK1 might be an upstream activator of Nrf2. To support this, overexpression of dominant-negative JNK1 suppressed Nrf2-induced ARE reporter gene expression in a dose-dependent manner. When PEITC was added, slight enhancement of ARE reporter gene expression was observed in either Nrf2- or JNK1-transfected cells. Finally, ARE reporter activity induced by PEITC was substantially attenuated by transfection of either dominant-negative mutant of Nrf2 or dominant-negative mutant of JNK1.
Conclusion. Taken together, these data suggest that JNK1 acts as an upstream activator of Nrf2 and that PEITC activates ARE-mediated phase II drug metabolism gene expressions via the JNK1- and Nrf2-dependent pathways.
Similar content being viewed by others
References
D. T. Verhoeven, H. Verhagen, R. A. Goldbohm, P. A. van den Brandt, and G. van Poppel. A review of mechanisms underlying anticarcinogenicity by brassica vegetables. Chem. Biol. Interact. 103:79–129 (1997).
P. Talalay and J. W. Fahey. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J. Nutr. 131:3027S–3033S (2001).
S. S. Hecht. Chemoprevention of cancer by isothiocyanates, modifiers of carcinogen metabolism. J. Nutr. 129:768S–774S (1999).
E. W. Boberg, E. C. Miller, J. A. Miller, A. Poland, and A. Liem. Strong evidence from studies with brachymorphic mice pentachlorophenol that 1′–sulfooxysafrole is the major ultimate electrophilic carcinogenic metabolite of 1′–hydroxysafrole in mouse liver. Cancer Res. 43:5163–5173 (1983).
K. Itoh, T. Chiba, S. Takahashi, T. Ishii, K. Igarashi, Y. Katoh, T. Oyake, N. Hayashi, K. Satoh, I. Hatayama, M. Yamamoto, and Y. Nabeshima. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 236:313–322 (1997).
T. Xie, M. Belinsky, Y. Xu, and A. K. Jaiswal. ARE–TRE–mediated regulation of gene expression. Response to xenobiotics antioxidants. J. Biol. Chem. 270:6894–6900 (1995).
T. H. Rushmore, M. R. Morton, and C. B. Pickett. The antioxidant responsive element. Activation by oxidative stress identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 266:11632–11639 (1991).
J. D. Hayes and M. McMahon. Molecular basis for the contribution of the antioxidant responsive element to cancer chemoprevention. Cancer Lett. 174:103–113 (2001).
R. Yu, W. Lei, S. Mandlekar, M. J. Weber, C. J. Der, J. Wu, and A. T. Kong. Role of a mitogen–activated protein kinase pathway in the induction of phase II detoxifying enzymes by chemicals. J. Biol. Chem. 274:27545–27552 (1999).
M. Ramos–Gomez, M. K. Kwak, P. M. Dolan, K. Itoh, M. Yamamoto, P. Talalay, and T. W. Kensler. Sensitivity to carcinogenesis is increased chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor–deficient mice. Proc. Natl. Acad. Sci. USA 98:3410–3415 (2001).
A. N. Kong, E. Owuor, R. Yu, V. Hebbar, C. Chen, R. Hu, and S. Mandlekar. Induction of xenobiotic enzymes by the MAP kinase pathway the antioxidant or electrophile response element (ARE/EpRE). Drug Metab. Rev. 33:255–271 (2001).
K. Itoh, N. Wakabayashi, Y. Katoh, T. Ishii, K. Igarashi, J. D. Engel, and M. Yamamoto. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino–terminal Neh2 domain. Genes Dev. 13:76–86 (1999).
R. Yu, C. Chen, Y. Y. Mo, V. Hebbar, E. D. Owuor, T. H. Tan, and A. N. Kong. Activation of mitogen–activated protein kinase pathways induces antioxidant response element–mediated gene expression via a Nrf2–dependent mechanism. J. Biol. Chem. 275:39907–39913 (2000).
H. C. Huang, T. Nguyen, and C. B. Pickett. Regulation of the antioxidant response element by protein kinase C–mediated phosphorylation of NF–E2–related factor 2. Proc. Natl. Acad. Sci. USA 97:12475–12480 (2000).
J. M. Lee, J. M. Hanson, W. A. Chu, and J. A. Johnson. Phosphatidylinositol 3–kinase, not extracellular signal–regulated kinase, regulates activation of the antioxidant–responsive element in IMR–32 human neuroblastoma cells. J. Biol. Chem. 276:20011–20016 (2001).
J. Jeyapaul and A. K. Jaiswal. Nrf2 c–Jun regulation of antioxidant response element (ARE)–mediated expression induction of gamma–glutamylcysteine synthetase heavy subunit gene. Biochem. Pharmacol. 59:1433–1439 (2000).
H. J. Prochaska and P. Talalay. Regulatory mechanisms of monofunctional bifunctional anticarcinogenic enzyme inducers in murine liver. Cancer Res. 48:4776–4782 (1988).
J. D. Brooks, V. G. Paton, and G. Vidanes. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiol. Biomarkers Prev. 10:949–954 (2001).
Y. Nakamura, H. Ohigashi, S. Masuda, A. Murakami, Y. Morimitsu, Y. Kawamoto, T. Osawa, M. Imagawa, and K. Uchida. Redox regulation of glutathione S–transferase induction by benzyl isothiocyanate: correlation of enzyme induction with the formation of reactive oxygen intermediates. Cancer Res. 60:219–225 (2000).
P. Rose, K. Faulkner, G. Williamson, and R. Mithen. 7–Methylsulfinylheptyl 8–methylsulfinyloctyl isothiocyanates from watercress are potent inducers of phase II enzymes. Carcinogenesis 21:1983–1988 (2000).
C. C. Conaway, D. Jiao, and F. L. Chung. Inhibition of rat liver cytochrome P450 isozymes by isothiocyanates their conjugates: a structure–activity relationship study. Carcinogenesis 17:2423–2427 (1996).
R. Yu, S. Mandlekar, K. J. Harvey, D. S. Ucker, and A. N. Kong. Chemopreventive isothiocyanates induce apoptosis caspase–3–like protease activity. Cancer Res. 58:402–408 (1998).
R. Yu, S. Mandlekar, W. Lei, W. E. Fahl, T. H. Tan, and A. T. Kong. p38 mitogen–activated protein kinase negatively regulates the induction of phase II drug–metabolizing enzymes that detoxify carcinogens. J. Biol. Chem. 275:2322–2327 (2000).
L. M. Zipper and R. T. Mulcahy. Inhibition of ERK p38 MAP kinases inhibits binding of Nrf2 induction of GCS genes. Biochem. Biophys. Res. Commun. 278:484–492 (2000).
A. T. Dinkova–Kostova, M. A. Massiah, R. E. Bozak, R. J. Hicks, and P. Talalay. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc. Natl. Acad. Sci. USA 98:3404–3409 (2001).
M. K. Kwak, K. Itoh, M. Yamamoto, and T. W. Kensler. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element–like sequences in the nrf2 promoter. Mol. Cell. Biol. 22:2883–2892 (2002).
Y. Zhang and P. Talalay. Mechanism of differential potencies of isothiocyanates as inducers of anticarcinogenic Phase 2 enzymes. Cancer Res. 58:4632–4639 (1998).
P. M. Chaudhary, M. T. Eby, A. Jasmin, and L. Hood. Activation of the c–Jun N–terminal kinase/stress–activated protein kinase pathway by overexpression of caspase–8 its homologs. J. Biol. Chem. 274:19211–19219 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keum, YS., Owuor, E.D., Kim, BR. et al. Involvement of Nrf2 and JNK1 in the Activation of Antioxidant Responsive Element (ARE) by Chemopreventive Agent Phenethyl Isothiocyanate (PEITC). Pharm Res 20, 1351–1356 (2003). https://doi.org/10.1023/A:1025737622815
Issue Date:
DOI: https://doi.org/10.1023/A:1025737622815