Abstract
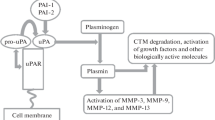
The urokinase type plasminogen activator (urokinase) plays a pivotal role in the regulation of cell adhesion and migration during tissue remodeling. Urokinase not only specifically cleaves plasminogen and converts it into plasmin but also activates intracellular signaling upon binding to certain receptors on the cell surface. The polyfunctional properties of this protein are associated with its three-domain structure as follows: the C-terminal proteolytic domain containing the serine protease active center, the central kringle domain, and the N-terminal domain homologous to epidermal growth factor. This review considers functional properties of urokinase and of its fragments generated on the cell surface as a result of proteolytic processing. This review will discuss the mechanisms of urokinase-mediated regulation of cellular function upon binding to membrane receptors.
Similar content being viewed by others
REFERENCES
Tkachuk, V., Stepanova, V., Little, P. J., and Bobik, A. (1996) Clin. Exp. Pharmacol. Physiol., 23, 759-765.
Clowes, A. W., Clowes, M. M., Au, Y. P. T., Reidy, M. A., and Belin, D. (1990) Circ. Res., 67, 61-67.
Eaton, D. L., Scott, R. W., and Baker, J. B. (1984) J. Biol. Chem., 259, 6241-6247.
Holmes, W. E., Pennica, D., Blaber, M., Rey, M. W., Guenzler, W. A., Steffens, G. L., and Heyneker, H. L. (1985) Biotechnology, 3, 923-929.
Appella, E., Robinson, E. A., Ullrich, S. J., Stoppelli, M. P., Corti, A., Cassani, G., and Blasi, F. (1987) J. Biol. Chem., 262, 4437-4440.
Mimuro, J., Kaneko, M., Murakami, T., Matsuda, M., and Sakata, Y. (1992) Biochim. Biophys. Acta, 1160, 325-334.
Stephens, R. W., Bokman, A. M., Myohanen, H. T., Reisberg, T., Tapiovaara, H., Pedersen, N., Gröndahl-Hansen, J., Llinás, M., and Vaheri, A. (1992) Biochemistry, 31, 7572-7579.
Mukhina, S., Stepanova, V., Traktouev, D., Poliakov, A., Beabealashvilly, R., Gursky, Ya., Minashkin. M., Shevelev, A., and Tkachuk, V. (2000) J. Biol. Chem., 275, 16450-16458.
Poliakov, A. A., Mukhina, S. A., Traktouev, D. O., Bibilashvily, R. S., Gursky, Y. G., Minashkin, M. M., Stepanova, V. V., and Tkachuk, V. A. (1999) J. Recept. Signal Transduct. Res., 19, 939-951.
Nowak, U. K., Li, X., Teuten, A. J., Smith, R. A., and Dobson, C. M. (1993) Biochemistry, 32, 298-309.
Bogusky, M. J., Dobson, C. M., and Smith, R. A. (1989) Biochemistry, 28, 6728-6735.
Kobayashi, H., Gotoh, J., Hirashima, Y., Fujie, M., Sugino, D., and Terao, T. (1995) J. Biol. Chem., 270, 8361-8366.
Lenich, C., Pannell, R., Henkin, J., and Gurewich, V. (1992) Thromb. Haemost., 68, 539-544.
Rabbani, S. A., Mazar, A. P., Bernier, S. M., Haq, M., Bolivar, I., Henkin, J., and Goltzman, D. (1992) J. Biol. Chem., 267, 14151-14156.
Nolan, C., Hall, L. S., Barlow, G. H., and Tribby, I. I. E. (1977) Biochim. Biophys. Acta, 496, 384-400.
Wun, T. C., Ossowski, L., and Reich, E. (1982) J. Biol. Chem., 257, 7262-7268.
Lijnen, H. R., Zamarron, C., Blaber, M., Winkler, M. E., and Collen, D. (1990) J. Biol. Chem., 265, 5232-5236.
Goldberg, G. I., Frisch, S. M., He, C., Wilhelm, S. M., Reich, R., and Collier, I. E. (1990) Ann. N. Y. Acad. Sci., 580, 375-384.
Stump, D. C., Lijnen, H. R., and Collen, D. (1986) J. Biol. Chem., 261, 17120-17126.
Stump, D. C., Thienpont, M., and Collen, D. (1986) J. Biol. Chem., 261, 1267-1273.
Kobayashi, O., Matsui, K., Minamiura, N., and Yamamoto, T. (1985) J. Biochem. (Tokyo), 97, 37-44.
Mazar, A. P., Buko, A., Petros, A., Barnathan, E. S., and Henkin, J. (1992) Fibrinolysis, 6(Suppl. 1), 49-55.
Poliakov, A., Tkachuk, V., Ovchinnikova, T., Potapenko, N., Bagryantsev, S., and Stepanova, V. (2001) Biochem. J., 355, 639-645.
Ichinose, A., Fujikawa, K., and Suyama, T. (1985) J. Biol. Chem., 261, 3486-3489.
Braat, E. A., Levi, M., Bos, R., Haverkate, F., Lassen, M. R., de Maat, M. P., and Rijken, D. C. (1999) J. Lab. Clin. Med., 134, 161-167.
Vassali, J.-D., Baccino, D., and Belin, D. (1985) J. Cell Biol., 100, 86-92.
Behrendt, N., Rønne, E., Ploug, M., Petri, T., Løber, D., Nielsen, L. S., Schleuning, W.-D., Blasi, F., Appella, E., and Danø, K. (1990) J. Biol. Chem., 265, 6453-6460.
Ploug, M., Rønne, E., Behrendt, N., Jensen, A. L., Blasi, F., and Danø, K. (1991) J. Biol. Chem., 266, 1926-1933.
Ploug, M. (1998) Biochemistry, 37, 16494-16505.
Quax, P. H., Grimbergen, J. M., Lansink, M., Bakker, A. H., Blatter, M. C., Belin, D., van Hinsbergh, V. W., and Verheijen, J. H. (1998) Arterioscler. Thromb. Vasc. Biol., 18, 693-701.
Ploug, M., Rahbek-Nielsen, H., Ellis, V., Roepstorff, P., and Dano, K. (1995) Biochemistry, 34, 12524-12534.
Magdolen, V., Rettenberger, P., Koppitz, M., Goretzki, L., Kessler, H., Weidle, U. H., Konig, B., Graeff, H., Schmitt, M., and Wilhelm, O. (1996) Eur. J. Biochem., 237, 743-751.
Shevach, E. M., and Korty, P. E. (1989) Immunol. Today, 10, 195-200.
Ploug, M., Kjalke, M., Ronne, E., Weidle, U., Hoyer-Hansen, G., and Dano, K. (1993) J. Biol. Chem., 268, 17539-17546.
Sugita, Y., Nakano, Y., Oda, E., Noda, K., Tobe, T., Miura, N. H., and Tomita, M. (1993) J. Biochem. (Tokyo), 114, 473-477.
Behrendt, N., Ploug, M., Patthy, L., Houen, G., Blasi, F., and Dano, K. (1991) J. Biol. Chem., 266, 7842-7847.
Ploug, M., Ellis, V., and Dano, K. (1994) Biochemistry, 33, 8991-8997.
Moller, L. B., Pollanen, J., Ronne, E., Pedersen, N., and Blasi, F. (1993) J. Biol. Chem., 268, 11152-11159.
Moller, L. B., Ploug, M., and Blasi, F. (1992) Eur. J. Biochem., 208, 493-500.
Andreasen, P. A., Kjoller, L., Christensen, L., and Duffy, M. J. (1997) Int. J. Cancer, 72, 1-22.
Monier, S., Parton, R. G., Vogel, F., Behlke, J., Henske, A., and Kurzchalia, T. V. (1995) Mol. Biol. Cell, 6, 911-927.
Ellis, V., Scully, M. F., and Kakkar, V. V. (1989) J. Biol. Chem., 264, 2185-2188.
Herz, J., Clouthier, D. E., and Hammer, R. E. (1992) Cell, 71, 411-421.
Conese, M., Olson, D., and Blasi, F. (1994) J. Biol. Chem., 269, 17886-17892.
Conese, M., Nykjaer, A., Petersen, C. M., Cremona, O., Pardi, R., Andreasen, P. A., Gliemann, J., Christensen, E. I., and Blasi, F. (1995) J. Cell Biol., 131, 1609-1622.
Argraves, K. M., Battey, F. D., MacCalman, C. D., McCrae, K. R., Gåfvels, M., Kozarsky, K. F., Chappell, D. A., Strauss, J. F., III, and Strickland, D. K. (1995) J. Biol. Chem., 270, 26550-26557.
Nykjaer, A., Conese, M., Christensen, E. I., Olson, D., Cremona, O., Gliemann, J., and Blasi, F. (1997) EMBO J., 16, 2610-2620.
Plouet, J., Moro, F., Bertagnolli, S., Coldeboeuf, N., Mazarguil, H., Clamens, S., and Bayard, F. (1997) J. Biol. Chem., 272, 13390-13396.
Saksela, O., and Rifkin, D. B. (1990) J. Cell Biol., 110, 767-775.
Dumler, I., Weis, A., Mayboroda, O. A., Maasch, C., Jerke, U., Haller, H., and Gulba, D. C. (1998) J. Biol. Chem., 273, 315-321.
Dumler, I., Kopmann, A., Weis, A., Mayboroda, O. A., Wagner, K., Gulba, D. C., and Haller, H. (1999) Arterioscler. Thromb. Vasc. Biol., 19, 290-297.
Resnati, M., Guttinger, M., Valcamonica, S., Sidenius, N., Blasi, F., and Fazioli, F. (1996) EMBO J., 15, 1572-1582.
Bohuslav, J., Horejší, V., Hansmann, C., Stöckl, J., Weidle, U. H., Majdic, O., Bartke, I., Knapp, W., and Stockinger, H. J. (1995) Exp. Med., 181, 1381-1390.
Konakova, M., Hucho, F., and Schleuning, W. D. (1998) Eur. J. Biochem., 253, 421-429.
Fazioli, F., Resnati, M., Sidenius, N., Higashimoto, Y., Appella, E., and Blasi, F. (1997) EMBO J., 16, 7279-7286.
Nguyen, D. H., Hussaini, I. M., and Gonias, S. L. (1998) J. Biol. Chem., 273, 8502-8507.
Tang, H., Kerins, D. M., Hao, Q., Inagami, T., and Vaughan, D. E. (1998) J. Biol. Chem., 273, 18268-18272.
Dumler, I., Petri, T., and Schleuning, W.-D. (1993) FEBS Lett., 322, 37-40.
Busso, N., Masur, S. K., Lazega, D., Waxman, S., and Ossowski, L. (1994) J. Cell Biol., 126, 259-270.
Cai, H., Erhardt, P., Troppmair, J., Diaz-Meco, M. T., Sithanandam, G., Rapp, U. R., Moscat, J., and Cooper, G. M. (1993) Mol. Cell. Biol., 13, 7645-7651.
Goretzki, L., and Mueller, B. M. (1998) Biochem. J., 336 (Pt. 2), 381-386.
Willnow, T. E., Nykjaer, A., and Herz, J. (1999) Nat. Cell Biol., 1, E157-E162.
Howell, B. W., Gertler, F. B., and Cooper, J. A. (1997) EMBO J., 16, 121-132.
Hiesberger, T., Trommsdorff, M., Howell, B. W., Goffinet, A., Mumby, M. C., Cooper, J. A., and Herz, J. (1999) Neuron, 24, 481-489.
Gotthardt, M., Trommsdorff, M., Nevitt, M. F., Shelton, J., Richardson, J. A., Stockinger, W., Nimpf, J., and Herz, J. (2000) J. Biol. Chem., 275, 25616-25624.
Goncharova, E. A., Tkachuk, V. A., Ratner, E. I., Parfyonova, Ye. V., and Vorotnikov, A. B. (2001) Zh. Evol. Biokhim. Fiziol., 36, 569-575.
Xue, W., Kindzelskii, A. L., Todd, R. F., III, and Petty, H. R. (1994) J. Immunol., 152, 4630-4640.
Sitrin, R. G., Pan, P. M., Harper, H. A., Todd, R. F., III, Harsh, D. M., and Blackwood, R. A. (2000) J. Immunol., 165, 3341-3349.
Kindzelskii, A. L., Laska, Z. O., Todd, R. F., III, and Petty, H. R. (1996) J. Immunol., 156, 297-309.
Xue, W., Mizukami, I., Todd, R. F., III, and Petty, H. R. (1997) Cancer Res., 57, 1682-1689.
Simon, D. I., Wei, Y., Zhang, L., Rao, N. K., Xu, H., Chen, Z., Liu, Q., Rosenberg, S., and Chapman, H. A. (2000) J. Biol. Chem., 275, 10228-10234.
Todd, R. F., and Petty, H. R. (1997) J. Lab. Clin. Med., 129, 492-498.
May, A. E., Kanse, S. M., Lund, L. R., Gisler, R. H., Imhof, B. A., and Preissner, K. T. (1998) J. Exp. Med., 188, 1029-1037.
Ghosh, S., Brown, R., Jones, J. C., Ellerbroek, S. M., and Stack, M. S. (2000) J. Biol. Chem., 275, 23869-23876.
Wei, Y., Yang, X., Liu, Q., Wilkins, J. A., and Chapman, H. A. (1999) J. Cell Biol., 144, 1285-1294.
Pollanen, J., Hedman, K., Nielsen, L. S., Dano, K., and Vaheri, A. (1988) J. Cell Biol., 106, 87-95.
Hebert, C. A., and Baker, J. B. (1988) J. Cell Biol., 106, 1241-1247.
Deng, G., Curriden, S. A., Wang, S., Rosenberg, S., and Loskutoff, D. J. (1996) J. Cell. Biol., 134, 1563-1571.
Hoyer-Hansen, G., Behrendt, N., Ploug, M., Dano, K., and Preissner, K. T. (1997) FEBS Lett., 420, 79-85.
Kanse, S. M., Kost, C., Wilhelm, O. G., Andreasen, P. A., and Preissner, K. T. (1996) Exp. Cell. Res., 224, 344-353.
Wei, Y., Waltz, D. A., Rao, N., Drummond, R. J., Rosenberg, S., and Chapman, H. A. (1994) J. Biol. Chem., 269, 32380-32388.
Dumler, I., Stepanova, V., Jerke, U., Mayboroda, O. A., Vogel, F., Bouvet, P., Tkachuk, V., Haller, H., and Gulba, D. C. (1999) Curr. Biol., 9, 1468-1476.
Manchanda, N., and Schwartz, B. S. (1995) J. Biol. Chem., 270, 20032-20035.
Reinartz, J., Schaefer, B., Bechtel, M. J., and Kramer, M. D. (1996) Exp. Cell Res., 223, 91-101.
Baker, J. B., Low, D. A., Simmer, R. L., and Cunningham, D. D. (1980) Cell, 21, 37-45.
Geiger, M., Huber, K., Wojta, J., Stingl, L., Espana, F., Griffin, J. H., and Binder, B. R. (1989) Blood, 74, 722-728.
Stefansson, S., Kounnas, M. Z., Henkin, J., Mallampalli, R. K., Chappell, D. A., Strickland, D. K., and Argraves, W. S. (1995) J. Cell Sci., 108, 2361-2368.
Kounnas, M. Z., Henkin, J., Argraves, W. S., and Strikland, D. K. (1993) J. Biol. Chem., 268, 21862-21867.
Willnow, T., Goldshtein, J. L., Orth, K., Brown, M., and Herz, J. (1992) J. Biol. Chem., 267, 26172-26180.
Orth, K., Willnow, T., Herz, J., Gething, M. J., and Sambrook, J. (1994) J. Biol. Chem., 269, 21117-21122.
Filippova, M. P., Bochkov, V. N., and Tkachuk, V. A. (1998) Usp. Biol. Khim., 38, 115-141.
Olson, D., Pollanen, J., Høyer-Hansen, G., Rønne, E., Sakaguchi, K., Wun, T.-Ch., Appella, E., Danø, K., and Blasi, F. (1992) J. Biol. Chem., 267, 9129-9133.
Cubellis, M. V., Wun, T.-C., and Blasi, F. (1990) EMBO J., 9, 1079-1085.
Nykjaer, A., Kjoller, L., Cohen, R. L., Lawrence, D. A., Garni-Wagner, B. A., Todd, R. F., van Zonneveld, A. J., Gliemann, J., and Andreasen, P. A. (1994) J. Biol. Chem., 269, 25668-25676.
Rodenburg, K. W., Kjoller, L., Petersen, H. H., and Andreasen, H. H. (1998) Biochem. J., 329 (Pt. 1), 55-63.
Stoppelli, M. P., Corti, A., Soffientini, A., Cassani, G., Blasi, F., and Assolian, R. K. (1985) Proc. Natl. Acad. Sci. USA, 82, 4939-4943.
Nykjaer, A., Petersen, C. M., Møller, B., Jensen, P. H., Moestrup, S. K., Holtet, T. L., Etzerodt, M., Thøgersen, H. C., Munch, M., Andreasen, P. A., and Gliemann, J. (1992) J. Biol. Chem., 267, 14543-14546.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stepanova, V.V., Tkachuk, V.A. Urokinase as a Multidomain Protein and Polyfunctional Cell Regulator. Biochemistry (Moscow) 67, 109–118 (2002). https://doi.org/10.1023/A:1013912500373
Issue Date:
DOI: https://doi.org/10.1023/A:1013912500373