Abstract
Introduction
In patients with advanced gastric cancer refractory to chemotherapy, the treatment options are limited. Via this phase II study, we aimed to assess the efficacy and safety of oxaliplatin in combination with 5-fluorouracil and l-leucovorin (modified FOLFOX6).
Methods
Patients who had histologically confirmed metastatic gastric cancer refractory to ≥ two previous chemotherapy regimens were included. The primary endpoint was the overall response rate (ORR) by an independent central review. According to an assumption of a threshold ORR of 10% and expected ORR of 25%, with α = 0.05 and β = 0.20, at least 33 patients were required. The secondary endpoints included overall survival (OS), progression-free survival (PFS), quality of life measured by EQ-5D, and safety.
Results
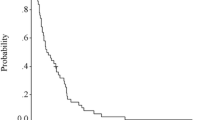
Among the 35 enrolled patients, 33 were included in the primary analysis. All patients previously received fluoropyrimidines, cisplatin, and taxanes, and 24 (73%) were pretreated with irinotecan. The confirmed ORR was 27% [95% confidence interval (CI) 13–46]. The median PFS and OS were 2.2 (95% CI 1.2–3.2) and 5.6 (95% CI 4.1–7.0) months, respectively. In the multivariate analyses, immunotherapy within 90 days and a Glasgow Prognostic Score of 0 were associated with better treatment outcomes. The most common grade ≥ 3 adverse event was neutropenia (36%), and no febrile neutropenia was observed. The median EQ-5D scores did not change from baseline at 2, 4, and 8 weeks (p value = 0.38, 0.79, and 0.98, respectively).
Conclusion
Modified FOLFOX6 (mFOLFOX6) showed substantial activity and acceptable toxicity for chemotherapy-refractory advanced gastric cancer.
Trial Registration
UMIN Clinical Trial Registry (UMIN000016416).


Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386386. https://doi.org/10.1002/ijc.29210.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines (5th edition). Gastric Cancer. 2020. https://doi.org/10.1007/s10120-020-01042-y.
Muro K, Van Cutsem E, Narita Y, Pentheroudakis G, Baba E, Li J, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer; a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS SSO and TOS. Ann Oncol. 2019;30:19–33. https://doi.org/10.1093/annonc/mdy502.
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–71. https://doi.org/10.1016/S0140-6736(17)31827-5.
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. https://doi.org/10.1001/jamaoncol.2018.0013.
Flynn M, Young K, Cunningham D, Starling N. The evolving immunotherapeutic landscape in advanced oesophagogastric cancer. Ther Adv Med Oncol. 2018;10:1758835918786228. https://doi.org/10.1177/1758835918786228.
Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:1437–48. https://doi.org/10.1016/s1470-2045(18)30739-3.
Perego P, Robert J. Oxaliplatin in the era of personalized medicine: from mechanistic studies to clinical efficacy. Cancer Chemother Pharmacol. 2016;77:5–18. https://doi.org/10.1007/s00280-015-2901-x.
Kondoh C, Kadowaki S, Komori A, Narita Y, Taniguchi H, Ura T, et al. Salvage chemotherapy with the combination of oxaliplatin, leucovorin, and 5-fluorouracil in advanced gastric cancer refractory or intolerant to fluoropyrimidines, platinum, taxanes, and irinotecan. Gastric Cancer. 2018;21:1050–7. https://doi.org/10.1007/s10120-018-0825-y.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
McMillan DC, Crozier JE, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22:881–6. https://doi.org/10.1007/s00384-006-0259-6.
Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. https://doi.org/10.1200/jco.2009.26.7609.
Osa A, Uenami T, Koyama S, Fujimoto K, Okuzaki D, Takimoto T, et al. Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients. JCI Insight. 2018. https://doi.org/10.1172/jci.insight.59125.
Tsuchiya A, Ikeda S, Ikegami N, Nishimura S, Sakai I, Fukuda T, et al. Estimating an EQ-5D population value set: the case of Japan. Health Econ. 2002;11:341–53. https://doi.org/10.1002/hec.673.
Kim DY, Kim JH, Lee SH, Kim TY, Heo DS, Bang YJ, et al. Phase II study of oxaliplatin, 5-fluorouracil and leucovorin in previously platinum-treated patients with advanced gastric cancer. Ann Oncol. 2003;14:383–7. https://doi.org/10.1093/annonc/mdg106.
Kim YS, Hong J, Sym SJ, Park SH, Park J, Cho EK, et al. Oxaliplatin, 5-fluorouracil and leucovorin (FOLFOX-4) combination chemotherapy as a salvage treatment in advanced gastric cancer. Cancer Res Treat. 2010;42:24–9. https://doi.org/10.4143/crt.2010.42.1.24.
Tsuji K, Yasui H, Onozawa Y, Boku N, Doyama H, Fukutomi A, et al. Modified FOLFOX-6 therapy for heavily pretreated advanced gastric cancer refractory to fluorouracil, irinotecan, cisplatin and taxanes: a retrospective study. Jpn J Clin Oncol. 2012;42:686–90. https://doi.org/10.1093/jjco/hys084.
Masuishi T, Kadowaki S, Kondo M, Komori A, Sugiyama K, Mitani S, et al. FOLFOX as first-line therapy for gastric cancer with severe peritoneal metastasis. Anticancer Res. 2017;37:7037–42. https://doi.org/10.21873/anticanres.12174.
Kim HS, Kim JH, Kim HJ, Jang HJ, Kim JB, Kim JW, et al. Oxaliplatin, 5-fluorouracil and leucovorin (modified FOLFOX-6) as first-line chemotherapy for advanced gastric cancer patients with poor performance status. Oncol Lett. 2012;3:425–8. https://doi.org/10.3892/ol.2011.496.
Tamayo E, Montes M, Vicente D, Perez-Trallero E. Streptococcus pyogenes pneumonia in adults: clinical presentation and molecular characterization of isolates 2006–2015. PLoS One. 2016;11:e0152640. https://doi.org/10.1371/journal.pone.0152640.
Schvartsman G, Peng SA, Bis G, Lee JJ, Benveniste MFK, Zhang J, et al. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer. 2017;112:90–5. https://doi.org/10.1016/j.lungcan.2017.07.034.
Pestana RC, Becnel M, Rubin ML, Torman DK, Crespo J, Phan J, et al. Response rates and survival to systemic therapy after immune checkpoint inhibitor failure in recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 2020;101:104523. https://doi.org/10.1016/j.oraloncology.2019.104523.
Park SE, Lee SH, Ahn JS, Ahn MJ, Park K, Sun JM. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thorac Oncol. 2018;13:106–11. https://doi.org/10.1016/j.jtho.2017.10.011.
Rossi S, Basso M, Strippoli A, Schinzari G, D'Argento E, Larocca M, et al. Are markers of systemic inflammation good prognostic indicators in colorectal cancer? Clin Colorectal Cancer. 2017;16:264–74. https://doi.org/10.1016/j.clcc.2017.03.015.
Acknowledgements
The authors thank Dr. M. Nomura from Kyoto University Graduate School of Medicine for tumor assessment. We also sincerely appreciate all the patients who received treatment and were enrolled in this study.
Funding
No funding or sponsorship was received for this study. The journal’s Rapid Service Fee was funded by the authors.
Editorial Assistance
The authors would like to thank Enago (www.enago.jp) for the English language review.
Authorship
All the authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Seiichiro Mitani reports payment for lectures from Taiho Pharmaceutical Co., Eli Lilly, Takeda Pharmaceutical Co., and Ono Pharmaceutical Co. Shigenori Kadowaki reports grants and payment for lectures from Eli Lilly and Taiho Pharmaceutical Co., grants from Ono Pharmaceutical Co. and Bristol-Myers Squibb, and payment for lectures from Yakult Honsha Co., Ltd., Chugai Pharmaceutical Co., Ltd., Bayer, and Merck. Chihiro Kondoh reports payment for lectures from Sanofi, Janssen Pharmaceutical K.K., NIPPON SHINYAKU, Eisai, MSD, Bristol-Myers Squibb, Chugai Pharmaceutical Co., and Takeda Pharmaceutical Co. Toshiki Masuishi reports grants from Daiichi Sankyo, MSD and Ono Pharmaceutical Co., and payment for lectures from Taiho Pharmaceutical Co., Chugai Pharmaceutical Co., Ltd., Eli Lilly, Takeda Pharmaceutical Co., Merck and Bayer. Yukiya Narita reports payment for lectures from Bristol-Myers Squibb, Ono Pharmaceutical Co., Nihon Kayaku and Taiho Pharmaceutical Co. Hiroya Taniguchi reports grants and payment for lectures from Takeda Pharmaceutical Co., grants from Daiichi Sankyo and Sysmex, and payment for lectures from Taiho Pharmaceutical Co., Chugai Pharmaceutical Co., Ltd., and Eli Lilly. Kei Muro reports grants and payment for lectures from Sanofi, grants from MSD, Daiichi Sankyo, Sumitomo Dainippon Pharma, Shionogi, Parexel International, Mediscience Planning and Pfizer, and payment for lectures from Taiho Pharmaceutical Co., Chugai Pharmaceutical Co., Ltd., Eli Lilly, Takeda Pharmaceutical Co., Ono Pharmaceutical Co., Bayer, and Bristol-Myers Squibb. All remaining authors (Azusa Komori, Isao Oze, Kyoko Kato, Kazunori Honda, Masashi Ando, Tsutomu Tanaka and Masahiro Tajika) have nothing to disclose.
Compliance with Ethics Guidelines
The protocol was approved by the institutional review board of Aichi Cancer Center Hospital. This study was approved by the institutional review boards and registered in the UMIN Clinical Trial Registry (UMIN000016416). Written informed consent was obtained from each patient before study enrollment, and the study was conducted in accordance with the Declaration of Helsinki.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12156927.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mitani, S., Kadowaki, S., Komori, A. et al. A Phase II Study of Modified FOLFOX6 for Advanced Gastric Cancer Refractory to Standard Therapies. Adv Ther 37, 2853–2864 (2020). https://doi.org/10.1007/s12325-020-01358-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01358-2