Abstract
Purpose of Review
Meningioma is a common intracranial neoplasm currently classified in 15 histologic subtypes across 3 grades of malignancy. First-choice therapy for meningioma is maximum safe resection for grade I tumors, and surgery plus optional and mandatory adjuvant radiotherapy for grade II and III, respectively, given the increased rate of recurrence even in the event of complete resection. The WHO 2016 histopathologic grading of meningioma has been questioned due to subjectivity and its controversial predictive power for recurrence.
Recent Findings
Novel DNA methylation profiling has simplified classification into six classes that seem to improve prognostic accuracy.
Summary
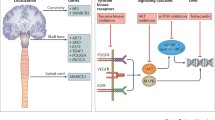
We review five main topics of molecular biology research regarding tumorigenesis and natural history of meningioma from the clinician’s perspective: the histopathologic diagnostic features and pitfalls of the current tumor classification; the molecular integrated diagnosis supported by identification of genetic alterations and DNA methylation profiling; the general landscape of the various signaling pathways involved in meningioma formation; the pathogenic theories of the peri-tumoral edema present in meningioma and its therapy implications; and a summarized review on the current treatments and plausible targeted therapies directed to meningioma. It seems likely that molecular assessment will be introduced within the next update of the WHO classification of meningiomas, acknowledging the promising value of DNA methylation profiling. This integrated diagnostic protocol will simplify tumor subtype categorization and provide improved accuracy in predicting recurrence and outcome. Although much effort is being done in identifying key gene mutations, and elucidating specific intracellular signaling pathways involved in meningioma tumorigenesis, effective targeted therapies for recurrent meningiomas are still lacking.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Marosi C, Hassler M, Roessler K, Reni M, Sant M, Mazza E, et al. Meningioma. Crit Rev Oncol Hematol. 2008;67(2):153–71. https://doi.org/10.1016/j.critrevonc.2008.01.010.
Pećina-Šlaus N, Kafka A, Lechpammer M. Molecular genetics of intracranial meningiomas with emphasis on canonical wnt signalling. Cancers (Basel). 2016;8(7):E67. https://doi.org/10.3390/cancers8070067.
Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. https://doi.org/10.1093/neuonc/noy131.
Whittle IR, Smith C, Navoo P, Collie D. Meningiomas. Lancet. 2004;363(9420):1535–43.
Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57(6):1088–95 discussion 1088-95.
Umansky F, Shoshan Y, Rosenthal G, Fraifeld S, Spektor S. Radiation-induced meningioma. Neurosurg Focus. 2008;24(5):E7. https://doi.org/10.3171/FOC/2008/24/5/E7.
Bowers DC, Moskowitz CS, Chou JF, Mazewski CM, Neglia JP, Armstrong GT, et al. Morbidity and mortality associated with meningioma after cranial radiotherapy: a report from the childhood cancer survivor study. J Clin Oncol. 2017;35(14):1570–6. https://doi.org/10.1200/JCO.2016.70.1896.
Kok JL, Teepen JC, van Leeuwen FE, Tissing WJE, Neggers SJCMM, van der Pal HJ, et al. Risk of benign meningioma after childhood cancer in the DCOG-LATER cohort: contributions of radiation dose, exposed cranial volume, and age. Neuro-Oncology. 2019;21(3):392–403. https://doi.org/10.1093/neuonc/noy124.
Goutagny S, Kalamarides M. Meningiomas and neurofibromatosis. J Neuro-Oncol. 2010;99(3):341–7. https://doi.org/10.1007/s11060-010-0339-x.
Perry A, Giannini C, Raghavan R, Scheithauer BW, Banerjee R, Margraf L, et al. Aggressive phenotypic and genotypic features in pediatric and NF2-associated meningiomas: a clinicopathologic study of 53 cases. J Neuropathol Exp Neurol. 2001;60(10):994–1003.
Blankenstein MA, Verheijen FM, Jacobs JM, Donker TH, van Duijnhoven MW, Thijssen JH. Occurrence, regulation, and significance of progesterone receptors in human meningioma. Steroids. 2000;65(10–11):795–800.
Muskens IS, Wu AH, Porcel J, Cheng I, Le Marchand L, Wiemels JL, et al. Body mass index, comorbidities, and hormonal factors in relation to meningioma in an ethnically diverse population: the Multiethnic Cohort. Neuro-Oncology. 2019;21(4):498–507. https://doi.org/10.1093/neuonc/noz005.
Wen PY, Quant E, Drappatz J, Beroukhim R, Norden AD. Medical therapies for meningiomas. J Neuro-Oncol. 2010;99(3):365–78. https://doi.org/10.1007/s11060-010-0349-8.
Ji Y, Rankin C, Grunberg S, Sherrod AE, Ahmadi J, Townsend JJ, et al. Double-blind phase III randomized trial of the antiprogestin agent mifepristone in the treatment of unresectable meningioma: SWOG S9005. J Clin Oncol. 2015;33(34):4093–8. https://doi.org/10.1200/JCO.2015.61.6490.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–20. https://doi.org/10.1007/s00401-016-1545-1.
Hale AT, Wang L, Strother MK, Chambless LB. Differentiating meningioma grade by imaging features on magnetic resonance imaging. J Clin Neurosci. 2018;48:71–5. https://doi.org/10.1016/j.jocn.2017.11.013.
Yang SY, Park CK, Park SH, Kim DG, Chung YS, Jung HW. Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry. 2008;79(5):574–80.
Kshettry VR, Ostrom QT, Kruchko C, Al-Mefty O, Barnett GH, Barnholtz-Sloan JS. Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro-Oncology. 2015;17(8):1166–73. https://doi.org/10.1093/neuonc/nov069.
Sughrue ME, Sanai N, Shangari G, Parsa AT, Berger MS, McDermott MW. Outcome and survival following primary and repeat surgery for World Health Organization Grade III meningiomas. J Neurosurg. 2010;113(2):202–9. https://doi.org/10.3171/2010.1.JNS091114.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109.
Bagshaw HP, Burt LM, Jensen RL, Suneja G, Palmer CA, Couldwell WT, et al. Adjuvant radiotherapy for atypical meningiomas. J Neurosurg. 2017;126(6):1822–8. https://doi.org/10.3171/2016.5.JNS152809.
•• Zhi M, Girvigian MR, Miller MJ, Chen JC, Schumacher AJ, Rahimian J, et al. Long-term outcomes of newly diagnosed resected atypical meningiomas and the role of adjuvant radiotherapy. World Neurosurg. 2019;122:e1153–61. https://doi.org/10.1016/j.wneu.2018.11.006. This paper reviews the prognosis of atypical meningioma and highlights the fact that atypical meningiomas are increasingly being diagnosed under modern criteria.
Rogers CL, Perry A, Pugh S, Vogelbaum MA, Brachman D, McMillan W, et al. Pathology concordance levels for meningioma classification and grading in NRG Oncology RTOG Trial 0539. Neuro-Oncology. 2016;18(4):565–74. https://doi.org/10.1093/neuonc/nov247.
Marciscano AE, Stemmer-Rachamimov AO, Niemierko A, Larvie M, Curry WT, Barker FG 2nd, et al. Benign meningiomas (WHO Grade I) with atypical histological features: correlation of histopathological features with clinical outcomes. J Neurosurg. 2016;124(1):106–14. https://doi.org/10.3171/2015.1.JNS142228.
•• Sahm F, Schrimpf D, Stichel D, DTW J, Hielscher T, Schefzyk S, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18(5):682–94. https://doi.org/10.1016/S1470-2045(17)30155-9. This seminal paper provides thorough information on the novel classification of meningiomas under epigenetic features, that is, methylation classes.
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–73. https://doi.org/10.1056/NEJMoa0808710.
Cancer Genome Atlas Research Network, Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade Gliomas. N Engl J Med. 2015;372(26):2481–98. https://doi.org/10.1056/NEJMoa1402121.
Pajtler KW, Witt H, Sill M, Jones DT, Hovestadt V, Kratochwil F, et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27(5):728–43. https://doi.org/10.1016/j.ccell.2015.04.002.
Hovestadt V, Remke M, Kool M, Pietsch T, Northcott PA, Fischer R, et al. Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta Neuropathol. 2013;125(6):913–6. https://doi.org/10.1007/s00401-013-1126-5.
•• BJA P, Oba-Shinjo SM, de Almeida AN, Marie SKN. Molecular alterations in meningiomas: Literature review. Clin Neurol Neurosurg. 2019;176:89–96. https://doi.org/10.1016/j.clineuro.2018.12.004. This paper provides a modern and thorough overview on the molecular alteration present in all types of meningioma.
Domingues P, González-Tablas M, Otero Á, Pascual D, Ruiz L, Miranda D, et al. Genetic/molecular alterations of meningiomas and the signaling pathways targeted. Oncotarget. 2015;6(13):10671–88.
Vaubel RA, Chen SG, Raleigh DR, Link MJ, Chicoine MR, Barani I, et al. Meningiomas with rhabdoid features lacking other histologic features of malignancy: a study of 44 cases and review of the literature. J Neuropathol Exp Neurol. 2016;75(1):44–52. https://doi.org/10.1093/jnen/nlv006.
Baumgarten P, Gessler F, Schittenhelm J, Skardelly M, Tews DS, Senft C, et al. Brain invasion in otherwise benign meningiomas does not predict tumor recurrence. Acta Neuropathol. 2016;132(3):479–81. https://doi.org/10.1007/s00401-016-1598-1.
Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383–91. https://doi.org/10.1016/S1470-2045(16)30321-7.
Hsu CC, Pai CY, Kao HW, Hsueh CJ, Hsu WL, Lo CP. Do aggressive imaging features correlate with advanced histopathological grade in meningiomas? J Clin Neurosci. 2010;17(5):584–7. https://doi.org/10.1016/j.jocn.2009.09.018.
Nagar VA, Ye JR, Ng WH, Chan YH, Hui F, Lee CK, et al. Diffusion-weighted MR imaging: diagnosing atypical or malignant meningiomas and detecting tumor dedifferentiation. AJNR Am J Neuroradiol. 2008;29(6):1147–52. https://doi.org/10.3174/ajnr.A0996.
Hwang WL, Marciscano AE, Niemierko A, Kim DW, Stemmer-Rachamimov AO, Curry WT, et al. Imaging and extent of surgical resection predict risk of meningioma recurrence better than WHO histopathological grade. Neuro-Oncology. 2016;18(6):863–72. https://doi.org/10.1093/neuonc/nov285.
Coroller TP, Bi WL, Huynh E, Abedalthagafi M, Aizer AA, Greenwald NF, et al. Radiographic prediction of meningioma grade by semantic and radiomic features. PLoS One. 2017;12(11):e0187908. https://doi.org/10.1371/journal.pone.0187908 eCollection 2017.
Ruttledge MH, Sarrazin J, Rangaratnam S, Phelan CM, Twist E, Merel P, et al. Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat Genet. 1994;6(2):180–4.
Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339(6123):1077–80. https://doi.org/10.1126/science.1233009.
Pham MH, Zada G, Mosich GM, Chen TC, Giannotta SL, Wang K, et al. Molecular genetics of meningiomas: a systematic review of the current literature and potential basis for future treatment paradigms. Neurosurg Focus. 2011;30(5):E7. https://doi.org/10.3171/2011.2.FOCUS1117.
Evans JJ, Jeun SS, Lee JH, Harwalkar JA, Shoshan Y, Cowell JK, et al. Molecular alterations in the neurofibromatosis type 2 gene and its protein rarely occurring in meningothelial meningiomas. J Neurosurg. 2001;94(1):111–7.
Brastianos PK, Horowitz PM, Santagata S, Jones RT, McKenna A, Getz G, et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45(3):285–9. https://doi.org/10.1038/ng.2526.
Clark VE, Harmancı AS, Bai H, Youngblood MW, Lee TI, Baranoski JF, et al. Recurrent somatic mutations in POLR2A define a distinct subset of meningiomas. Nat Genet. 2016;48(10):1253–9. https://doi.org/10.1038/ng.3651.
Abedalthagafi M, Bi WL, Aizer AA, Merrill PH, Brewster R, Agarwalla PK, et al. Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma. Neuro-Oncology. 2016;18(5):649–55. https://doi.org/10.1093/neuonc/nov316.
Bi WL, Mei Y, Agarwalla PK, Beroukhim R, Dunn IF. Genomic and epigenomic landscape in meningioma. Neurosurg Clin N Am. 2016;27(2):167–79. https://doi.org/10.1016/j.nec.2015.11.009.
Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010;20(3):161–8. https://doi.org/10.1016/j.semcancer.2010.05.002.
Huang Q, Zhao SL, Tian XY, Li B, Li Z. Increased co-expression of macrophage migration inhibitory factor and matrix metalloproteinase 9 is associated with tumor recurrence of meningioma. Int J Med Sci. 2013;10(3):276–85. https://doi.org/10.7150/ijms.5185.
Karsy M, Azab MA, Abou-Al-Shaar H, Guan J, Eli I, Jensen RL, et al. Clinical potential of meningioma genomic insights: a practical review for neurosurgeons. Neurosurg Focus. 2018;44(6):E10. https://doi.org/10.3171/2018.2.FOCUS1849.
Yuzawa S, Nishihara H, Tanaka S. Genetic landscape of meningioma. Brain Tumor Pathol. 2016;33(4):237–47.
Pavelin S, Bečić K, Forempoher G, Tomić S, Capkun V, Drmić-Hofman I, et al. The significance of immunohistochemical expression of merlin, Ki-67, and p53 in meningiomas. Appl Immunohistochem Mol Morphol. 2014;22(1):46–9. https://doi.org/10.1097/PAI.0b013e318289f490.
Abbritti RV, Polito F, Cucinotta M, Lo Giudice C, Caffo M, Tomasello C, et al. Meningiomas and Proteomics: Focus on New Potential Biomarkers and Molecular Pathways. Cancer Genomics Proteomics. 2016;13(5):369–79.
Mawrin C, Sasse T, Kirches E, Kropf S, Schneider T, Grimm C, et al. Different activation of mitogen-activated protein kinase and Akt signaling is associated with aggressive phenotype of human meningiomas. Clin Cancer Res. 2005;11(11):4074–82.
James MF, Han S, Polizzano C, Plotkin SR, Manning BD, Stemmer-Rachamimov AO, et al. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009;29(15):4250–61. https://doi.org/10.1128/MCB.01581-08.
Boström J, Meyer-Puttlitz B, Wolter M, Blaschke B, Weber RG, Lichter P, et al. Alterations of the tumor suppressor genes CDKN2A (p16(INK4a)), p14(ARF), CDKN2B (p15(INK4b)), and CDKN2C (p18(INK4c)) in atypical and anaplastic meningiomas. Am J Pathol. 2001;159(2):661–9.
Hou J, Kshettry VR, Selman WR, Bambakidis NC. Peritumoral brain edema in intracranial meningiomas: the emergence of vascular endothelial growth factor-directed therapy. Neurosurg Focus. 2013;35(6):E2. https://doi.org/10.3171/2013.8.FOCUS13301.
Berhouma M, Jacquesson T, Jouanneau E, Cotton F. Pathogenesis of peri-tumoral edema in intracranial meningiomas. Neurosurg Rev. 2019;42(1):59–71. https://doi.org/10.1007/s10143-017-0897-x.
Vignes JR, Sesay M, Rezajooi K, Gimbert E, Liguoro D. Peritumoral edema and prognosis in intracranial meningioma surgery. J Clin Neurosci. 2008;15(7):764–8. https://doi.org/10.1016/j.jocn.2007.06.001.
Probst-Cousin S, Villagran-Lillo R, Lahl R, Bergmann M, Schmid KW, Gullotta F. Secretory meningioma: clinical, histologic, and immunohistochemical findings in 31 cases. Cancer. 1997;79(10):2003–15.
de Vries J, Wakhloo AK. Cerebral oedema associated with WHO-I, WHO-II, and WHO-III-meningiomas: correlation of clinical, computed tomographic, operative and histological findings. Acta Neurochir. 1993;125(1–4):34–40.
Simis A, Pires de Aguiar PH, Leite CC, Santana PA Jr, Rosemberg S, Teixeira MJ. Peritumoral brain edema in benign meningiomas: correlation with clinical, radiologic, and surgical factors and possible role on recurrence. Surg Neurol. 2008;70(5):471–7; discussion 477. https://doi.org/10.1016/j.surneu.2008.03.006.
Nakasu S, Fukami T, Jito J, Matsuda M. Microscopic anatomy of the brain-meningioma interface. Brain Tumor Pathol. 2005;22(2):53–7.
Nakano T, Asano K, Miura H, Itoh S, Suzuki S. Meningiomas with brain edema: radiological characteristics on MRI and review of the literature. Clin Imaging. 2002;26(4):243–9.
Klatzo I. Evolution of brain edema concepts. Acta Neurochir Suppl (Wien). 1994;60:3–6.
Bitzer M, Topka H, Morgalla M, Friese S, Wöckel L, Voigt K. Tumor-related venous obstruction and development of peritumoral brain edema in meningiomas. Neurosurgery. 1998;42(4):730–7.
Tanaka M, Imhof HG, Schucknecht B, Kollias S, Yonekawa Y, Valavanis A. Correlation between the efferent venous drainage of the tumor and peritumoral edema in intracranial meningiomas: superselective angiographic analysis of 25 cases. J Neurosurg. 2006;104(3):382–8.
Smith HP, Challa VR, Moody DM, Kelly DL Jr. Biological features of meningiomas that determine the production of cerebral edema. Neurosurgery. 1981;8(4):428–33.
Ding YS, Wang HD, Tang K, Hu ZG, Jin W, Yan W. Expression of vascular endothelial growth factor in human meningiomas and peritumoral brain areas. Ann Clin Lab Sci. 2008;38(4):344–51.
Markovic M, Antunovic V, Milenkovic S, Zivkovic N. Prognostic value of peritumoral edema and angiogenesis in intracranial meningioma surgery. J BUON. 2013;18(2):430–6.
Pistolesi S, Fontanini G, Camacci T, De Ieso K, Boldrini L, Lupi G, et al. Meningioma-associated brain oedema: the role of angiogenic factors and pial blood supply. J Neuro-Oncol. 2002;60(2):159–64.
Paek SH, Kim DG, Park CK, Phi JH, Kim YY, Im SY, et al. The role of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinase in microcystic meningiomas. Oncol Rep. 2006;16(1):49–56.
Reszec J, Hermanowicz A, Rutkowski R, Turek G, Mariak Z, Chyczewski L. Expression of MMP-9 and VEGF in meningiomas and their correlation with peritumoral brain edema. Biomed Res Int. 2015;2015:646853–8. https://doi.org/10.1155/2015/646853.
Wang P, Ni RY, Chen MN, Mou KJ, Mao Q, Liu YH. Expression of aquaporin-4 in human supratentorial meningiomas with peritumoral brain edema and correlation of VEGF with edema formation. Genet Mol Res. 2011;10(3):2165–71. https://doi.org/10.4238/vol10-3gmr1212.
Roth P, Happold C, Weller M. Corticosteroid use in neuro-oncology: an update. Neurooncol Pract. 2015;2(1):6–12.
Puchner MJ, Hans VH, Harati A, Lohmann F, Glas M, Herrlinger U. Bevacizumab-induced regression of anaplastic meningioma. Ann Oncol. 2010;21(12):2445–6. https://doi.org/10.1093/annonc/mdq634.
Nayak L, Iwamoto FM, Rudnick JD, Norden AD, Lee EQ, Drappatz J, et al. Atypical and anaplastic meningiomas treated with bevacizumab. J Neuro-Oncol. 2012;109(1):187–93. https://doi.org/10.1007/s11060-012-0886-4.
Black PM, Villavicencio AT, Rhouddou C, Loeffler JS. Aggressive surgery and focal radiation in the management of meningiomas of the skull base: preservation of function with maintenance of local control. Acta Neurochir. 2001;143(6):555–62.
Buerki RA, Horbinski CM, Kruser T, Horowitz PM, James CD, Lukas RV. An overview of meningiomas. Future Oncol. 2018;14(21):2161–77. https://doi.org/10.2217/fon-2018-0006.
Euskirchen P, Peyre M. Management of meningioma. Presse Med. 2018;47(11–12 Pt 2):e245–52. https://doi.org/10.1016/j.lpm.2018.05.016.
Rogers L, Barani I, Chamberlain M, Kaley TJ, McDermott M, Raizer J, et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122(1):4–23. https://doi.org/10.3171/2014.7.JNS131644.
Sun SQ, Hawasli AH, Huang J, Chicoine MR, Kim AH. An evidence-based treatment algorithm for the management of WHO grade II and III meningiomas. Neurosurg Focus. 2015;38(3):E3. https://doi.org/10.3171/2015.1.FOCUS14757.
Paldor I, Awad M, Sufaro YZ, Kaye AH, Shoshan Y. Review of controversies in management of non-benign meningioma. J Clin Neurosci. 2016;31:37–46. https://doi.org/10.1016/j.jocn.2016.03.014.
Bailo M, Gagliardi F, Boari N, Castellano A, Spina A, Mortini P. The role of surgery in meningiomas. Curr Treat Options Neurol. 2019;21(10):51. https://doi.org/10.1007/s11940-019-0587-9.
Nanda A, Bir SC, Konar S, Maiti TK, Bollam P. World Health Organization Grade I convexity meningiomas: study on outcomes, complications and recurrence rates. World Neurosurg. 2016;89:620–627.e2. https://doi.org/10.1016/j.wneu.2015.11.050.
Jenkinson MD, Javadpour M, Haylock BJ, Young B, Gillard H, Vinten J, et al. The ROAM/EORTC-1308 trial: Radiation versus Observation following surgical resection of Atypical Meningioma: study protocol for a randomised controlled trial. Trials. 2015;16:519. https://doi.org/10.1186/s13063-015-1040-3.
Delgado-López PD, Corrales-García EM. Role of adjuvant radiotherapy in atypical (WHO grade II) and anaplastic (WHO grade III) meningiomas: a systematic review. Clin Transl Oncol. 2020. https://doi.org/10.1007/s12094-020-02434-3.
Grunberg SM, Weiss MH, Russell CA, Spitz IM, Ahmadi J, Sadun A, et al. Long-term administration of mifepristone (RU486): clinical tolerance during extended treatment of meningioma. Cancer Investig. 2006;24(8):727–33.
Scerrati A, Mongardi L, Visani J, Lofrese G, Cavallo MA, Fiorentino A, et al. The controversial role of Bevacizumab in the treatment of patients with intracranial meningioma: a comprehensive literature review. Expert Rev Anticancer Ther. 2020;6:1–7. https://doi.org/10.1080/14737140.2020.1736567.
Graillon T, Romano D, Defilles C, Saveanu A, Mohamed A, Figarella-Branger D, et al. Octreotide therapy in meningiomas: in vitro study, clinical correlation, and literature review. J Neurosurg. 2017;127(3):660–9. https://doi.org/10.3171/2016.8.JNS16995.
Gupta S, Bi WL, Dunn IF. Medical management of meningioma in the era of precision medicine. Neurosurg Focus. 2018;44(4):E3. https://doi.org/10.3171/2018.1.FOCUS17754.
Preusser M, Brastianos PK, Mawrin C. Advances in meningioma genetics: novel therapeutic opportunities. Nat Rev Neurol. 2018;14(2):106–15. https://doi.org/10.1038/nrneurol.2017.168.
Kaley TJ, Wen P, Schiff D, Ligon K, Haidar S, Karimi S, et al. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro-Oncology. 2015;17(1):116–21. https://doi.org/10.1093/neuonc/nou148.
Choy W, Kim W, Nagasawa D, Stramotas S, Yew A, Gopen Q, et al. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus. 2011;30(5):E6. https://doi.org/10.3171/2011.2.FOCUS1116.
Mawrin C. Animal models of meningiomas. Chin Clin Oncol. 2017;6(Suppl 1):S6. https://doi.org/10.21037/cco.2017.05.03.
Touat M, Idbaih A, Sanson M, Ligon KL. Glioblastoma targeted therapy: updated approaches from recent biological insights. Ann Oncol. 2017;28(7):1457–72. https://doi.org/10.1093/annonc/mdx106.
Author information
Authors and Affiliations
Contributions
Pedro D. Delgado-López had the idea for the article, Delgado-López and Martín-Alonso performed the literature search and initial analysis, Delgado-López drafted the article, all authors critically revised the manuscript and all authors approved the final version.
Corresponding author
Ethics declarations
Conflict of Interest
Pedro David Delgado-López, Esther Cubo-Delgado, Jerónimo Javier González Bernal, and Javier Martín-Alonso each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuro-Oncology
Rights and permissions
About this article
Cite this article
Delgado-López, P.D., Cubo-Delgado, E., González-Bernal, J.J. et al. A Practical Overview on the Molecular Biology of Meningioma. Curr Neurol Neurosci Rep 20, 62 (2020). https://doi.org/10.1007/s11910-020-01084-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s11910-020-01084-w