Abstract
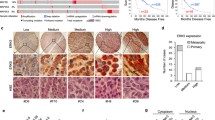
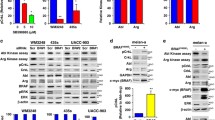
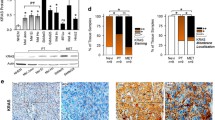
Data regarding the expression of epidermal growth factor receptor (EGFR) in melanoma and its role in the tumor biology are conflicting. In BRAF V600-mutant melanomas, the expression of EGFR has been associated with acquired resistance to BRAF inhibitors. In this study, we assessed EGFR expression and downstream signaling activity in a panel of melanoma cell lines and we investigated the effects of the BRAF inhibitor vemurafenib on expression of EGFR and its downstream effectors in a subgroup of BRAF-mutant melanoma cells. Three out of 10 melanoma cell lines expressed EGFR. Downstream signaling via ERK and AKT was responsive to either stimulation by EGF or inhibition by erlotinib. Constitutive activation of ERK occurred in all the cell lines investigated whereas constitutive activation of AKT only in three cell lines. Constitutive activation of ERK and AKT was independent from EGFR expression. Vemurafenib did not affect EGFR expression in general, but it increased EGFR phosphorylation in the cell line SkMel5. Induced EGFR phosphorylation was sensitive to treatment with erlotinib. Vemurafenib efficiently blocked ERK activation in all the BRAF-mutant cell lines tested, whereas its effects on AKT activation were dissimilar in the different cell lines. Our data suggest that EGFR is functional but usually inactive in EGFR high-expressing cell lines. Basal EGFR expression unlikely represents a biomarker for predicting the sensitivity to vemurafenib in melanoma, but EGFR activation might represent a mechanism of vemurafenib resistance in a subset of melanoma cells.





Similar content being viewed by others
References
Nicholson RI, Gee JM, Harper ME, EGFR and cancer prognosis (2001) Eur J Cancer 37(4):S9–S15
Lee CK, Brown C, Gralla RJ, Hirsh V, Thongprasert S, Tsai CM, Tan EH, Ho JC, da Chu T, Zaatar A et al (2013) Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 105:595–605
Liao BC, Lin CC, Yang JC (2013) First-line management of EGFR-mutated advanced lung adenocarcinoma: recent developments. Drugs 73:357–369
Real FX, Rettig WJ, Chesa PG, Melamed MR, Old LJ, Mendelsohn J (1986) Expression of epidermal growth factor receptor in human cultured cells and tissues: relationship to cell lineage and stage of differentiation. Cancer Res 46:4726–4731
Sparrow LE, Heenan PJ (1999) Differential expression of epidermal growth factor receptor in melanocytic tumours demonstrated by immunohistochemistry and mRNA in situ hybridization. Australas J Dermatol 40:19–24
Grahn JC, Isseroff RR (2004) Human melanocytes do not express EGF receptors. J Invest Dermatol 123:244–246
Elder DE, Rodeck U, Thurin J, Cardillo F, Clark WH, Stewart R, Herlyn M (1989) Antigenic profile of tumor progression stages in human melanocytic nevi and melanomas. Cancer Res 49:5091–5096
De Wit PE, Moretti S, Koenders PG, Weterman MA, van Muijen GN, Gianotti B, Ruiter DJ (1992) Increasing epidermal growth factor receptor expression in human melanocytic tumor progression. J Invest Dermatol 99:168–173
Rákosy Z, Vízkeleti L, Ecsedi S, Vokó Z, Bégány A, Barok M, Krekk Z, Gallai M, Szentirmay Z, Adány R et al (2007) EGFR gene copy number alterations in primary cutaneous malignant melanomas are associated with poor prognosis. Int J Cancer 121:1729–1737
Boone B, Jacobs K, Ferdinande L, Taildeman J, Lambert J, Peeters M, Bracke M, Pauwels P, Brochez L (2011) EGFR in melanoma: clinical significance and potential therapeutic target. J Cutan Pathol 38:492–502
Mirmohammadsadegh A, Mota R, Gustrau A, Hassan M, Nambiar S, Marini A, Bojar H, Tannapfel A, Hengge UR (2007) ERK1/2 is highly phosphorylated in melanoma metastases and protects melanoma cells from cisplatin-mediated apoptosis. J Invest Dermatol 127:2207–2215
Djerf EA, Trinks C, Abdiu A, Thunell LK, Hallbeck A-L, Walz TM (2009) ErbB receptor tyrosine kinases contribute to proliferation of malignant melanoma cells: inhibition by gefitinib (ZD1839). Melanoma Res 19:156–166
Patel SP, Kim KB, Papadopoulos NE, Hwu W-J, Hwu P, Prieto VG, Bar-Eli M, Zigler M, Dobroff A, Bronstein Y et al (2011) A phase II study of gefitinib in patients with metastatic melanoma. Melanoma Res 21:357–363
Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K et al (2010) Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 363:809–819
Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT et al (2012) Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 366:707–714
Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, Beijersbergen RL, Bardelli A, Bernards R (2012) Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 483:100–103
Keilholz U, Goldin-Lang P, Bechrakis NE, Max N, Letsch A, Schmittel A, Scheibenbogen C, Heufelder K, Eggermont A, Thiel E (2004) Quantitative detection of circulating tumor cells in cutaneous and ocular melanoma and quality assessment by real-time reverse transcriptase-polymerase chain reaction. Clin Cancer Res 10:1605–1612
Schicher N, Paulitschke V, Swoboda A, Kunstfeld R, Loewe R, Pilarski P, Pehamberger H, Hoeller C (2009) Erlotinib and bevacizumab have synergistic activity against melanoma. Clin Cancer Res 15:3495–3502
Bracher A, Cardona AS, Tauber S, Fink AM, Steiner A, Pehamberger H, Niederleithner H, Petzelbauer P, Gröger M, Loewe R (2013) Epidermal growth factor facilitates melanoma lymph node metastasis by influencing tumor lymphangiogenesis. J Invest Dermatol 133:230–238
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W et al (2002) Mutations of the BRAF gene in human cancer. Nature 417:949–954
Bennett DC (2008) How to make a melanoma: what do we know of the primary clonal events? Pigment Cell Melanoma Res 21:27–38
Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J et al (2010) COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 468:968–972
Su F, Bradley WD, Wang Q, Yang H, Xu L, Higgins B, Kolinsky K, Packman K, Kim MJ, Trunzer K et al (2012) Resistance to selective BRAF inhibition can be mediated by modest upstream pathway activation. Cancer Res 72:969–978
Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, Brown RD, Della Pelle P, Dias-Santagata D, Hung KE et al (2012) EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov 2:227–235
Girotti MR, Pedersen M, Sanchez-Laorden B, Viros A, Turajlic S, Niculescu-Duvaz D, Zambon A, Sinclair J, Hayes A, Gore M et al (2013) Inhibiting EGF receptor or SRC family kinase signaling overcomes BRAF inhibitor resistance in melanoma. Cancer Discov 3:158–167
Held MA, Langdon CG, Platt JT, Graham-Steed T, Liu Z, Chakraborty A, Bacchiocchi A, Koo A, Haskins JW, Bosenberg MW et al (2013) Genotype-selective combination therapies for melanoma identified by high-throughput drug screening. Cancer Discov 3:52–67
Rodriguez-Lopez AM, Smyth T, Curry J, Graham B, McMenamin R, Lyons J Abstract 2772 (2012). AT13387, an HSP90 inhibitor, is effective in both vemurafenib-sensitive and -resistant melanoma models. Cancer Res 72:2772–2772
Atefi M, von Euw E, Attar N, Ng C, Chu C, Guo D, Nazarian R, Chmielowski B, Glaspy JA, Comin-Anduix B et al (2011) Reversing melanoma cross-resistance to BRAF and MEK inhibitors by co-targeting the AKT/mTOR pathway. PLoS One 6:e28973
Jiang CC, Lai F, Thorne RF, Yang F, Liu H, Hersey P, Zhang XD (2011) MEK-independent survival of B-RAFV600E melanoma cells selected for resistance to apoptosis induced by the RAF inhibitor PLX4720. Clin Cancer Res 17:721–730
Vergani E, Vallacchi V, Frigerio S, Deho P, Mondellini P, Perego P, Cassinelli G, Lanzi C, Testi MA, Rivoltini L et al (2011) Identification of MET and SRC activation in melanoma cell lines showing primary resistance to PLX4032. Neoplasia 13:1132–1142
Sánchez-Hernández I, Baquero P, Calleros L, Chiloeches A (2012) Dual inhibition of (V600E)BRAF and the PI3K/AKT/mTOR pathway cooperates to induce apoptosis in melanoma cells through a MEK-independent mechanism. Cancer Lett 314:244–255
Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H et al (2010) Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 468:973–977
Won J-K, Yang HW, Shin S-Y, Lee JH, Heo WD, Cho K-H (2012) The crossregulation between ERK and PI3K signaling pathways determines the tumoricidal efficacy of MEK inhibitor. J Mol Cell Biol 4:153–163
Yuen H-F, Abramczyk O, Montgomery G, Chan K-K, Huang Y-H, Sasazuki T, Shirasawa S, Gopesh S, Chan KW, Fennell D et al (2012) Impact of oncogenic driver mutations on feedback between the PI3K and MEK pathways in cancer cells. Biosci Rep 32:413–422
Conflicts of Interest
All authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Cellular localization of EGFR. Five human melanoma cell lines were stained with a fluorescent antibody against EGFR. To evaluate extracellular EGFR expression, cells were stained directly after scratching and washing. For intracellular staining, cells were fixed and permeabilized prior to incubation with the fluorescent antibody. The human squamous cell carcinoma cell line FaDu served as positive control (GIF 59 kb)
Rights and permissions
About this article
Cite this article
Gross, A., Niemetz-Rahn, A., Nonnenmacher, A. et al. Expression and activity of EGFR in human cutaneous melanoma cell lines and influence of vemurafenib on the EGFR pathway. Targ Oncol 10, 77–84 (2015). https://doi.org/10.1007/s11523-014-0318-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-014-0318-9