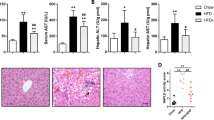
Abstract
High fructose flux enhances hepatocellular triglyceride accumulation (hepatic steatosis), which is a prime trigger in the emergence of hepatic ailments. Nevertheless, the pathophysiology underlying the process is not completely understood. Emerging evidences have revealed the inputs from multiple cues including inflammation, oxidative stress, and endoplasmic reticulum (ER) stress in the development of hepatic steatosis. Here, we substantiated the role of NLRP3 inflammasome and its convergence with oxidative and ER stress leading to hepatic steatosis under high fructose diet feeding. Male SD rats were fed on 60% high fructose diet (HFrD) for 10 weeks and treated with antioxidant quercetin or NLRP3 inflammasome inhibitor glyburide during the last 6 weeks, followed by metabolic characterization and analysis of hepatic parameters. HFrD-induced hepatic steatosis was associated with the activation of NLRP3 inflammasome, pro-inflammatory response, oxidative, and ER stress in liver. Treatment with quercetin abrogated HFrD-induced oxidative stress, along with attenuation of NLRP3 activation in the liver. On the other hand, inhibition of NLRP3 signaling by glyburide suppressed HFrD-induced oxidative and ER stress. Both glyburide or quercetin treatment significantly attenuated hepatic steatosis, associated with mitigated expression of the lipogenic markers in liver. Our findings verified the association of NLRP3 inflammasome with oxidative and ER stress in fructose-induced lipogenic response and indicate that in addition to be a target of oxidative/ER stress, NLRP3 can act as a trigger for oxidative/ER stress to activate a vicious cycle where these cues act in a complex manner to propagate inflammatory response, leading to hepatic steatosis.






Similar content being viewed by others
Availability of Data and Materials
All the materials and data reported in the manuscript are available with the corresponding author (AK Tamrakar).
References
Sanders, F.W.B., and J.L. Griffin. 2016. De novo lipogenesis in the liver in health and disease: More than just a shunting yard for glucose. Biological reviews of the Cambridge Philosophical Society 91: 452–468.
Lim, J.S., M. Mietus-Snyder, A. Valente, J.M. Schwarz, and R.H. Lustig. 2010. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nature Reviews Gastroenterology & Hepatology 7: 251–264.
Sanyal, A.J. 2019. Past, present and future perspectives in nonalcoholic fatty liver disease. Nature Reviews Gastroenterology & Hepatology 16: 377–386.
Hu, Y., I. Semova, X. Sun, H. Kang, S. Chahar, A.N. Hollenberg, D. Masson, M.D. Hirschey, J. Miao, and S.B. Biddinger. 2018. Fructose and glucose can regulate mammalian target of rapamycin complex 1 and lipogenic gene expression via distinct pathways. Journal of Biological Chemistry 293: 2006–2014.
Arrese, M., D. Cabrera, A.M. Kalergis, and A.E. Feldstein. 2016. Innate immunity and inflammation in NAFLD/NASH. Digestive Diseases and Sciences 61: 1294–1303.
Zhang, X., J.H. Zhang, X.Y. Chen, Q.H. Hu, M.X. Wang, R. Jin, Q.Y. Zhang, W. Wang, R. Wang, L.L. Kang, J.S. Li, M. Li, Y. Pan, J.J. Huang, and L.D. Kong. 2015. Reactive oxygen species-induced TXNIP drives fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxidant & Redox Signalling 22: 848–870.
Wan, X., C. Xu, C. Yu, and Y. Li. 2016. Role of NLRP3 inflammasome in the progression of NAFLD to NASH. Canadian Journal of Gastroenterology and Hepatology 2016: 6489012.
Kelley, N., D. Jeltema, Y. Duan, and Y. He. 2019. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. International Journal of Molecular Sciences 20: 3328.
Sharma, M., and E.D. Alba. 2021. Structure, activation and regulation of NLRP3 and AIM2 inflammasomes. International Journal of Molecular Sciences 22: 872.
Yu, X., L.P. Ren, C. Wang, Y.J. Zhu, H.Y. Xing, J. Zhao, and G.Y. Song. 2018. Role of X-box binding protein-1 in fructose-induced de novo lipogenesis in HepG2 cells. Chinese Medical Journal (Engl). 131: 2310–2319.
Malhi, H., and R.J. Kaufman. 2011. Endoplasmic reticulum stress in liver disease. Journal of Hepatology 54: 795–809.
Takaki, A., D. Kawai, and K. Yamamoto. 2013. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). International Journal of Molecular Sciences 14: 20704–20728.
Minutoli, L., D. Puzzolo, M. Rinaldi, N. Irrera, H. Marini, V. Arcoraci, A. Bitto, G. Crea, A. Pisani, F. Squadrito, V. Trichilo, D. Bruschetta, A. Micali, and D. Altavilla. 2016. ROS-mediated NLRP3 inflammasome activation in brain, heart, kidney, and testis ischemia/reperfusion injury. Oxidative Medicine and Cellular Longevity 2016: 2183026.
Bauernfeind, F., E. Bartok, A. Rieger, L. Franchi, G. Nunez, and V. Hornung. 2011. Cutting edge: Reactive oxygen species inhibitors block priming, but not activation, of the nlrp3 inflammasome. Journal of Immunology 187: 613–617.
Munoz-Planillo, R., P. Kuffa, G. Martinez-Colon, B.L. Smith, T.M. Rajendiran, and G. Nunez. 2013. K+ efflux is the common trigger of nlrp3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38: 1142–1153.
Zhou, Y., Z. Tong, S. Jiang, W. Zheng, J. Zhao, and X. Zhou. 2020. The roles of endoplasmic reticulum in NLRP3 inflammasome activation. Cells 9: 1219.
Buchanan, B.W., A.B. Mehrtash, C.L. Broshar, A.M. Runnebohm, B.J. Snow, L.N. Scanameo, M. Hochstrasser, and E.M. Rubenstein. 2019. Endoplasmic reticulum stress differentially inhibits endoplasmic reticulum and inner nuclear membrane protein quality control degradation pathways. Journal of Biological Chemistry 294: 19814–19830.
Kim, S., Y. Joe, S.O. Jeong, M. Zheng, S.H. Back, S.W. Park, S.W. Ryter, and H.T. Chung. 2014. Endoplasmic reticulum stress is sufficient for the induction of IL-1b production via activation of the NF-jB and inflammasome pathways. Innate Immunity 20: 799–815. https://doi.org/10.1177/1753425913508593.
Bronner, D.N., B.H. Abuaita, X. Chen, K.A. Fitzgerald, G. Nuñez, Y. He, X.M. Yin, and M.X.D. O’Riordan. 2015. Endoplasmic reticulum stress activates the inflammasome via NLRP3-and caspase-2-driven mitochondrial damage. Immunity 43: 451–462.
Collino, M., E. Benetti, M. Rogazzo, R. Mastrocola, M.M. Yaqoob, M. Aragno, et al. 2013. Reversal of the deleterious effects of chronic dietary HFCS-55 intake by PPAR-δ agonism correlates with impaired NLRP3 inflammasome activation. Biochemical Pharmacology 85: 257–264.
Gupta, A.P., P. Singh, R. Garg, G.R. Valicherla, M. Riyazuddin, A.A. Syed, Z. Hossain, and J.R. Gayen. 2019. Pancreastatin inhibitor activates AMPK pathway via GRP78 and ameliorates dexamethasone induced fatty liver disease in C57BL/6 mice. Biomedicine & Pharmacotherapy 116: 108959.
Verma, D.K., S. Gupta, J. Biswas, N. Joshi, A. Singh, P. Gupta, S. Tiwari, K.S. Raju, S. Chaturvedi, M. Wahajuddin, and S. Singh. 2018. New therapeutic activity of metabolic enhancer piracetam in treatment of neurodegenerative disease: Participation of caspase independent death factors, oxidative stress, inflammatory responses and apoptosis. BBA Molecular Basis of Disease 1864: 2078–2096.
Bagul, P.K., H. Middela, S. Matapally, R. Padiya, T. Bastia, K. Madhusudana, B.R. Reddy, S. Chakravarty, and S.K. Banerjee. 2012. Attenuation of insulin resistance, metabolic syndrome and hepatic oxidative stress by resveratrol in fructose-fed rats. Pharmacological Research 66: 260–268.
Ding, X.Q., W.Y. Wu, R.Q. Jiao, T.T. Gu, Q. Xu, Y. Pan, and L.D. Kong. 2018. Curcumin and allopurinol ameliorate fructose-induced hepatic inflammation in rats via miR-200a-mediated TXNIP/NLRP3 inflammasome inhibition. Pharmacological Research. 137: 64–75.
Le, K.A., and L. Tappy. 2006. Metabolic effects of fructose. Current Opinion in Clinical Nutrition and Metabolic Care 9: 469–475.
Jaiswal, N., C.K. Maurya, J. Pandey, A.K. Rai, and A.K. Tamrakar. 2015. Fructose-induced ROS generation impairs glucose utilization in L6 skeletal muscle cells. Free Radical Research 49: 1055–1068.
Hetz, C. 2012. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nature Reviews Molecular Cell Biology 13: 89–102.
Lu, J., and A. Holmgren. 2013. The thioredoxin antioxidant system. Free Radical Biology and Medicine 66: 75–87.
Zhou, R., A. Tardivel, B. Thorens, I. Choi, and J. Tschopp. 2010. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nature Immunology. 11: 136–140.
Zahid, A., B. Li, A.J.K. Kombe, T. Jin, and J. Tao. 2019. Pharmacological inhibitors of the NLRP3 inflammasome. Frontiers in Immunology 10: 2538.
Kim, G.N., and H.D. Jang. 2009. Protective mechanism of quercetin and rutin using glutathione metabolism on HO-induced oxidative stress in HepG2 cells. Annals of the New York Academy of Sciences 1171: 530–537.
Harijith, A., D.L. Ebenezer, and V. Natarajan. 2014. Reactive oxygen species at the crossroads of inflammasome and inflammation. Frontiers in Physiology 5: 352.
Zhou, R., A.S. Yazdi, P. Menu, and J. Tschopp. 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225.
Todoric, J., G. Di Caro, S. Reibe, D.C. Henstridge, C.R. Green, A. Vrbanac, F. Ceteci, C. Conche, R. McNulty, S. Shalapour, K. Taniguchi, P.J. Meikle, J.D. Watrous, R. Moranchel, et al. 2020. Fructose stimulated de novo lipogenesis is promoted by inflammation. Nature Metabolism 2: 1034–1045.
Nomura, K., and T. Yamanouchi. 2012. The role of fructose-enriched diets in mechanisms of nonalcoholic fatty liver disease. Journal of Nutritional Biochemistry 23: 203–208.
Choe, J.Y., and S.K. Kim. 2017. Quercetin and ascorbic acid suppress fructose-induced NLRP inflammasome activation by blocking intracellular shuttling of TXNIP in human macrophage cell lines. Inflammation 40: 980–994.
Hotamisligil, G.S. 2010. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140: 900–917.
Lebeaupin, C., E. Proics, C.H. de Bieville, D. Rousseau, S. Bonnafous, S. Patouraux, G. Adam, V.J. Lavallard, C. Rovere, O. Le Thuc, M.C. Saint-Paul, R. Anty, A.S. Schneck, A. Iannelli, J. Gugenheim, A. Tran, P. Gual, and B. Bailly-Maitre. 2015. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Disease 6: e1879.
Chong, W.C., M.D. Shastri, and R. Eri. 2017. Endoplasmic reticulum stress and oxidative stress: A vicious nexus implicated in bowel disease pathophysiology. International Journal of Molecular Sciences 18: 771–780.
Zhang, C., X. Chen, R.M. Zhu, Y. Zhang, T. Yu, H. Wang, H. Zhao, M. Zhao, Y.L. Ji, Y.H. Chen, X.H. Meng, W. Wei, and D.X. Xu. 2012. Endoplasmic reticulum stress is involved in hepatic SREBP-1c activation and lipid accumulation in fructose-fed mice. Toxicology Letters 212: 229–240.
Cho, I.J., D.H. Oh, J. Yoo, Y.C. Hwang, K.J. Ahn, H.Y. Chung, S.W. Jeong, J.Y. Moon, S.H. Lee, S.J. Lim, and I.K. Jeong. 2021. Allopurinol ameliorates high fructose diet induced hepatic steatosis in diabetic rats through modulation of lipid metabolism, inflammation, and ER stress pathway. Scientific Reports 11: 9894.
Wang, W., C. Wang, X.Q. Ding, Y. Pan, T.T. Gu, M.X. Wang, Y.L. Liu, F.M. Wang, S.J. Wang, and L.D. Kong. 2013. Quercetin and allopurinol reduce liver thioredoxin-interacting protein to alleviate inflammation and lipid accumulation in diabetic rats. British Journal of Pharmacology 169: 1352–1371.
Lerner, A.G., J.P. Upton, P.V. Praveen, R. Ghosh, Y. Nakagawa, A. Igbaria, S. Shen, V. Nguyen, B.J. Backes, M. Heiman, et al. 2012. Ire1alpha induces thioredoxin-interacting protein to activate the nlrp3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metabolism 16: 250–264.
Zhang, Y., X. Qu, H. Gao, J. Zhai, L. Tao, J. Sun, Y. Song, and J. Zhang. 2020. Quercetin attenuates NLRP3 inflammasome activation and apoptosis to protect INH-induced liver injury via regulating SIRT1 pathway. International Immunopharmacology 85: 106634.
Chanjitwiriya, K., S. Roytrakul, and D. Kunthalert. 2020. Quercetin negatively regulates IL-1beta production in Pseudomonas aeruginosa-infected human macrophages through the inhibition of MAPK/NLRP3 inflammasome pathways. PLoS ONE 15: e0237752.
Hu, Q.H., X. Zhang, Y. Pan, Y.C. Li, and L.D. Kong. 2012. Allopurinol, quercetin and rutin ameliorate renal NLRP3 inflammasome activation and lipid accumulation in fructose-fed rats. Biochemical Pharmacology 84: 113–125.
Yang, X., C. Qu, J. Jia, and Y. Zhan. 2019. NLRP3 inflammasome inhibitor glyburide expedites diabetic-induced impaired fracture healing. Immunobiology 224: 786–791.
Liu, L., Y. Dong, M. Ye, S. Jin, J. Yang, M.E. Joosse, Y. Sun, J. Zhang, M. Lazarev, S.R. Brant, B. Safar, M. Marohn, E. Mezey, and X. Li. 2017. The pathogenic role of NLRP3 inflammasome activation in inflammatory bowel diseases of both mice and humans. Journal of Crohn’s and Colitis 11: 737–750.
Acknowledgements
Authors would like to acknowledge funding support by the grant (EMR/2017/000936) from Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India. SS, AS, SA, FG, and PK acknowledge the financial support in the form of Research Fellowship from the Council of Scientific and Industrial Research (CSIR), New Delhi. This manuscript bears the CDRI communication No. 10438.
Funding
This work was supported by the grant (EMR/2017/000936) from Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by S. Singh, A. Sharma, B. Guru, S. Ahmad, F. Gulzar, I. Ahmad, and P. Kumar. Conceptualization, supervision, and data analysis were performed by A.K. Tamrakar. The first draft of the manuscript was written by S. Singh and A.K. Tamrakar and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Animal experimental protocol reported in the study was approved by the Institutional Animal Ethics Committee (IAEC) of the CSIR-Central Drug Research Institute, Lucknow, and work with the animals was conducted in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, S., Sharma, A., Ahmad, S. et al. Convergence of Fructose-Induced NLRP3 Activation with Oxidative Stress and ER Stress Leading to Hepatic Steatosis. Inflammation 46, 217–233 (2023). https://doi.org/10.1007/s10753-022-01727-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-022-01727-9