Abstract
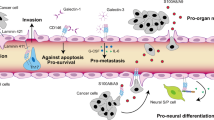
The dynamic interaction between tumor cells and their microenvironment induces a proinflammatory milieu that drives cancer development and progression. The S100A8/A9 complex has been implicated in chronic inflammation, tumor development, and progression. The cancer microenvironment contributes to the up-regulation of this protein complex in many invasive tumors, which is associated with the formation of pre-metastatic niches and poor prognosis. Changing adhesive preference of cancer cells is at the core of the metastatic process that governs the reciprocal interactions of cancer cells with the extracellular matrices and neighboring stromal cells. Cell adhesion molecules (CAMs) have been confirmed to have high-level expression in various highly invasive tumors. The expression and function of CAMs are profoundly influenced by the extracellular milieu. S100A8/A9 mediates its effects by binding to cell surface receptors, such as heparan sulfate, TLR4 and RAGE on immune and tumor cells. RAGE has recently been identified as an adhesion molecule and has considerably high identity and similarity to ALCAM and MCAM, which are frequently over-expressed on metastatic malignant melanoma cells. In this study, we demonstrated that ALCAM and MCAM also function as S100A8/A9 receptors as does RAGE and induce malignant melanoma progression by NF-κB activation and ROS formation. Notably, MCAM not only activated NF-κB more prominently than ALCAM and RAGE did but also mediated intracellular signaling for the formation of lung metastasis. MCAM is known to be involved in malignant melanoma development and progression through several mechanisms. Therefore, MCAM is a potential effective target in malignant melanoma treatment.









Similar content being viewed by others
Abbreviations
- MCAM:
-
Melanoma cell adhesion molecule
- ALCAM:
-
Activated leukocyte cell adhesion molecule
- RAGE,:
-
Receptor for advanced glycation end products
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
References
Greten FR et al (2004) IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118:285–296
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357:539–545
Ruegg C (2006) Leukocytes, inflammation, and angiogenesis in cancer: fatal attractions. J Leukoc Biol 80:682–684
Gebhardt C, Breitenbach U, Tuckermann JP et al (2002) Calgranulins S100A8 and S100A9 are negatively regulated by glucocorticoids in a c-Fos-dependent manner and overexpressed throughout skin carcinogenesis. Oncogene 21:4266–4276
Ott HW, Lindner H, Sarg B et al (2003) Calgranulins in cystic fluid and serum from patients with ovarian carcinomas. Cancer Res 63:7507–7514
Gebhardt C, Nemeth J, Angel P et al (2006) S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol 72:1622–1631
Salama I, Malone PS, Mihaimeed F et al (2008) A review of the S100 proteins in cancer. Eur J Surg Oncol 34:357–364
Kerkhoff C, Voss A, Scholzen TE et al (2012) Novel insights into the role of S100A8/A9 in skin biology. Exp Dermatol 21:822–826
Arai K, Takano S, Teratani T (2008) S100A8 and S100A9 overexpression is associated with poor pathological parameters in invasive ductal carcinoma of the breast. Curr Cancer Drug Targets 8:243–252
Katz AB, Taichman LB (1999) A partial catalog of proteins secreted by epidermal keratinocytes in culture. J Invest Dermatol 112:818–821
Thorey IS, Roth J, Regenbogen J et al (2001) The Ca2+ -binding proteins S100A8 and S100A9 are encoded by novel injury-regulated genes. J Biol Chem 276:35818–35825
Foell D, Frosch M, Sorg C et al (2004) Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta 344:37–51
Robinson MJ, Tessier P, Poulsom R et al (2002) The S100 family heterodimer, MRP-8/14, binds with high affinity to heparin and heparan sulfate glycosaminoglycans on endothelial cells. J Biol Chem 277:3658–3665
Vogl T, Tenbrock K, Ludwig S et al (2007) Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 13:1042–1049
Srikrishna G, Panneerselvam K, Westphal V et al (2001) Two proteins modulating transendothelial migration of leukocytes recognize novel carboxylated glycans on endothelial cells. J Immunol 166:4678–4688
Ghavami S, Rashedi I, Dattilo BM et al (2008) S100A8/A9 at low concentration promotes tumor cell growth via RAGE ligation and MAP kinase-dependent pathway. J Leukoc Biol 83:1484–1492
Turovskaya O, Foell D, Sinha P et al (2008) RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis 29:2035–2043
Ichikawa M, Williams R, Wang L et al (2011) S100A8/A9 activate key genes and pathways in colon tumor progression. Mol Cancer Res 9:133–148
Yin C, Li H, Zhang B et al (2013) RAGE-binding S100A8/A9 promotes the migration and invasion of human breast cancer cells through actin polymerization and epithelial–mesenchymal transition. Breast Cancer Res Treat 142:297–309
Hermani A, De Servi B, Medunjanin S et al (2006) S100A8 and S100A9 activate MAP kinase and NF-kappaB signaling pathways and trigger translocation of RAGE in human prostate cancer cells. Exp Cell Res 312:184–197
Chen B, Miller AL, Rebelatto M et al (2015) S100A9 Induced Inflammatory Responses Are Mediated by Distinct Damage Associated Molecular Patterns (DAMP) Receptors In Vitro and In Vivo. PLoS ONE 10:e0115828
Albelda SM (1993) Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest 68:4–17
Al-Mehdi AB, Tozawa K, Fisher A et al (2000) Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med 6:100–101
Pollard TD, Earnshaw WC (2002) Cellular adhesion. Cell biology, 6th edn. Saunders, Philadelphia, p 507–524
Yang Y, Jun CD, Liu JH et al (2004) Structural basis for dimerization of ICAM-1 on the cell surface. Mol Cell 14:269–276
Sessa L, Gatti E, Zeni F et al (2014) The receptor for advanced glycation end-products (RAGE) is only present in mammals, and belongs to a family of cell adhesion molecules (CAMs). PLoS ONE 9:e86903
Holzmann B, Brbcker EB, Lehmann JM et al (1987) Tumor progression in human malignant melanoma: five stages defined by their antigenic phenotypes. Int J Cancer 39:466–471
Luca M, Hunt B, Bucana CD et al (1993) Direct correlation between MUC18 expression and metastatic potential of human melanoma cells. Melanoma Res 3:35–41
Degen W, van Kempen L, Gijzen E et al (1998) MEMD, a new cell adhesion molecule in metastasizing human melanoma cell lines is identical to ALCAM (activated leukocyte cell adhesion molecule). Am J Pathol 152:805–813
van Kempen LC, van den Oord JJ, van Muijen GN et al (2000) Activated leukocyte cell adhesion molecule/CD166, a marker of tumor progression in primary malignant melanoma of the skin. Am J Pathol 156:769–774
Kristiansen G, Pilarsky C, Wissmann C et al (2005) Expression profiling of microdissected matched prostate cancer samples reveals CD166/MEMD and CD24 as new prognostic markers for patient survival. J Pathol 205:359–376
King JA, Ofori-Acquah SF, Stevens T et al (2004) Activated leukocyte cell adhesion molecule in breast cancer: Prognostic indicator. Breast Cancer Res 6:478–487
Davies SR, Dent C, Watkins G et al (2008) Expression of the cell to cell adhesion molecule, ALCAM, in breast cancer patients and the potential link with skeletal metastasis. Oncol Rep 19:555–561
Weichert W, Knosel T, Bellach J et al (2004) ALCAM/CD166 is overexpressed in colorectal carcinoma and correlates with shortened patient survival. J Clin Pathol 57:1160–1164
Verma A, Shukla NK, Deo SV et al (2005) MEMD/ALCAM: a potential marker for tumor invasion and nodal metastasis in esophageal squamous cell carcinoma. Oncology 68:462–470
Wang Z, Yan X (2013) CD146, a multi-functional molecule beyond adhesion. Cancer Lett 330:150–162
Wang J, Tang X, Weng W et al (2015) The membrane protein melanoma cell adhesion molecule (MCAM) is a novel tumor marker that stimulates tumorigenesis in hepatocellular carcinoma. Oncogene 34:5781–5795
Sakaguchi M, Watanabe M, Kinoshita R et al (2014) Dramatic increase in expression of a transgene by insertion of promoters downstream of the cargo gene. Mol Biotechnol 56:621–630
Sakaguchi M, Murata H, Aoyama Y et al (2014) DNAX-activating protein 10 (DAP10) membrane adaptor associates with receptor for advanced glycation end products (RAGE) and modulates the RAGE-triggered signaling pathway in human keratinocytes. J Biol Chem 289:23389–23402
Bowen MA, Patel DD, Li X et al (1995) Cloning, mapping, and characterization of activated leukocyte-cell adhesion molecule (ALCAM), a CD6 ligand. J Exp Med 181:2213–2220
Gebhardt C, Riehl A, Durchdewald M et al (2008) RAGE signaling sustains inflammation and promotes tumor development. J Exp Med 205:275–285
Iotzova-Weiss G, Dziunycz PJ, Freiberger SN et al (2015) S100A8/A9 Stimulates keratinocyte proliferation in the development of squamous cell carcinoma of the skin via the receptor for advanced glycation-end products. PLoS ONE 10:e0120971
Sunahori K, Yamamura M, Yamana J et al (2006) The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthr Res Ther 8:R69
Meyskens FL Jr, Buckmeier JA, McNulty SE et al (1999) Activation of nuclear factor-kappa B in human metastatic melanoma cells and the effect of oxidative stress. Clin Cancer Res 5:1197–1202
Fried L, Arbiser JL (2008) The reactive oxygen-driven tumor: relevance to melanoma. Pigment Cell Melanoma Res 21:117–122
Zhang Y, Du Y, Le W et al (2011) Redox control of the survival of healthy and diseased cells. Antioxid Redox Signal 15:2867–2908. doi:10.1089/ars.2010.3685
Wu WS (2006) The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev 25:695–705
Rapanotti MC, Suarez Viguria TM, Costanza G et al (2014) Sequential molecular analysis of circulating MCAM/MUC18 expression: a promising disease biomarker related to clinical outcome in melanoma. Arch Dermatol Res 306:527–537
Lehmann JM, Holzmann B, Breitbart EW et al (1987) Discrimination between benign and malignant cells of melanocytic lineage by two novel antigens, a glycoprotein. with a molecular weight of 113,000 and a protein with a molecular weight of 76,000. Cancer Res 47:841–845
Hiratsuka S, Watanabe A, Aburatani H et al (2006) Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol 8:1369–1375
Ang CW, Nedjadi T, Sheikh AA et al (2010) Smad4 loss is associated with fewer S100A8-positive monocytes in colorectal tumors and attenuated response to S100A8 in colorectal and pancreatic cancer cells. Carcinogenesis 31:1541–1551
Saha A, Lee YC, Zhang Z et al (2010) Lack of an endogenous anti-inflammatory protein in mice enhances colonization of B16F10 melanoma cells in the lungs. J Biol Chem 285:10822–10831
Sinha P, Okoro C, Foell D et al (2008) Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol 181:4666–4675
Wang L, Chang EW, Wong SC et al (2013) Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. J Immunol 190:794–804
Karin M, Cao Y, Greten FR et al (2002) NF-kappa B in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2:301–310
Morgan MJ, Liu ZG (2011) Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res 21:103–115
Mehdi MZ, Azar ZM, Srivastava AK (2007) Role of receptor and nonreceptor protein tyrosine kinases in H2O2-induced PKB and ERK1/2 signaling. Cell Biochem Biophys 47:1–10
Yamamoto Y, Gaynor RB (2001) Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest 107:135–142
Luo Y, Ellis LZ, Dallaglio K et al (2012) Side population cells from human melanoma tumors reveal diverse mechanisms for chemoresistance. J Invest Dermatol 132:2440–2450
Hibino T, Sakaguchi M, Miyamoto S et al (2013) S100A9 is a novel ligand of EMMPRIN that promotes melanoma metastasis. Cancer Res 73:172–183
Ribe A, McNutt NS (2003) S100A protein expression in the distinction between lentigo maligna and pigmented actinic keratosis. Am J Dermatopathol 25:93–99
Wu GJ, Fu P, Wang SW, Wu MW (2008) Enforced expression of MCAM/MUC18 increases in vitro motility and invasiveness and in vivo metastasis of two mouse melanoma K1735 sublines in a syngeneic mouse model. Mol Cancer Res 11:1666–1677
Mirkina I, Hadzijusufovic E, Krepler C et al (2014) Phenotyping of human melanoma cells reveals a unique composition of receptor targets and a subpopulation co-expressing ErbB4 EPO-R and NGF-R. PLoS ONE 1:e84417
Ishikawa T, Wondimu Z, Oikawa Y et al (2014) Laminins 411 and 421 differentially promote tumor cell migration via alpha6beta1 integrin and MCAM (CD146). Matrix Biol 38:69–83
Rapanotti MC, Ricozzi I, Campione E et al (2013) Blood MUC-18/MCAM expression in patients with melanoma: a suitable marker of poor outcome. Br J Dermatol 169:221–222
Rao C, Bui T, Connelly M et al (2011) Circulating melanoma cells and survival in metastatic melanoma. Int J Oncol 38:755–760
Khoja L, Lorigan P, Zhou C et al (2013) Biomarker utility of circulating tumor cells in metastatic cutaneous melanoma. J Invest Dermatol 133:1582–1590
Ordonez NG (2014) Value of melanocytic-associated immunohistochemical markers in the diagnosis of malignant melanoma: a review and update. Hum Pathol 2:191–205
Klinac D, Gray ES, Freeman JB et al (2014) Monitoring changes in circulating tumour cells as a prognostic indicator of overall survival and treatment response in patients with metastatic melanoma. BMC Cancer 14:423
Mills L, Tellez C, Huang S et al (2002) Fully human antibodies to MCAM/MUC18 inhibit tumor growth and metastasis of human melanoma. Cancer Res 62:5106–5114
Yan X, Lin Y, Yang D et al (2003) A novel anti-CD146 monoclonal antibody, AA98, inhibits angiogenesis and tumor growth. Blood 102:184–191
Prosen L, Markelc B, Dolinsek T et al (2014) Mcam silencing with RNA interference using magnetofection has antitumor effect in murine melanoma. Mol Ther Nucleic Acids 3:e205
Kang Y, Massague J (2004) Epithelial–mesenchymal transitions: twist in development and metastasis. Cell 118:277–279
Todorovic V, Sersa G, Cemazar M (2013) Gene electro-transfer of siRNAs against CD146 inhibits migration and invasion of human malignant melanoma cells SK-MEL28. Cancer Gene Ther 20:208–210
Wu Z, Wu Z, Li J et al (2012) MCAM is a novel metastasis marker and regulates spreading, apoptosis and invasion of ovarian cancer cells. Tumour Biol 33:1619–1628
Zabouo G, Imbert AM, Jacquemier J et al (2009) CD146 expression is associated with a poor prognosis in human breast tumors and with enhanced motility in breast cancer cell lines. Breast Cancer Res 11:R1
Chen W, Zhang HL, Jiang YG et al (2009) Inhibition of CD146 gene expression via RNA interference reduces in vitro perineural invasion on ACC-M cell. J Oral Pathol Med 38:198–205
Bu P, Zhuang J, Feng J et al (2007) Visualization of CD146 dimerization and its regulation in living cells. Biochim Biophys Acta 1773:513–520
McGary EC, Lev DC, Bar-Eli M (2002) Cellular adhesion pathways and metastatic potential of human melanoma. Cancer Biol Ther 5:459–465
Melnikova VO, Balasubramanian K, Villares GJ et al (2009) Crosstalk between protease-activated receptor 1 and platelet-activating factor receptor regulates melanoma cell adhesion molecule (MCAM/MUC18) expression and melanoma metastasis. J Biol Chem 284:28845–28855
Yazawa EM, Geddes-Sweeney JE, Cedeno-Laurent F et al (2015) Melanoma cell galectin-1 ligands functionally correlate with malignant potential. J Invest Dermatol 135:1849–1862
Tu T, Zhang C, Yan H et al (2015) CD146 acts as a novel receptor for netrin-1 in promoting angiogenesis and vascular development. Cell Res 25:275–287
Ye Z, Zhang C, Tu T et al (2013) Wnt5a uses CD146 as a receptor to regulate cell motility and convergent extension. Nat Commun 4:2803
Acknowledgments
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grant–in-Aid for Scientific Research (B), No. 26,290,039; Grant–in-Aid for Challenging Exploratory Research, No. 15K14382) (M. Sakaguchi), from the Takeda Science Foundation (M. Sakaguchi), from the Princess Takamatsu Cancer Research Fund (14-24613; M. Sakaguchi), and from the Kobayashi Foundation for Cancer Research (M. Sakaguchi).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ruma, I.M.W., Putranto, E.W., Kondo, E. et al. MCAM, as a novel receptor for S100A8/A9, mediates progression of malignant melanoma through prominent activation of NF-κB and ROS formation upon ligand binding. Clin Exp Metastasis 33, 609–627 (2016). https://doi.org/10.1007/s10585-016-9801-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-016-9801-2