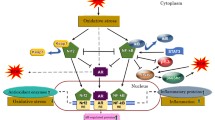
Abstract
p66Shc, a 66 kDa proto-oncogene Src homologous-collagen homologue (Shc) adaptor protein, is classically known in mediating receptor tyrosine kinase signaling and recently identified as a sensor to oxidative stress-induced apoptosis and as a longevity protein in mammals. The expression of p66Shc is decreased in mice and increased in human fibroblasts upon aging and in aging-related diseases, including prostate cancer. p66Shc protein level correlates with the proliferation of several carcinoma cells and can be regulated by steroid hormones. Recent advances point that p66Shc protein plays a role in mediating cross-talk between steroid hormones and redox signals by serving as a common convergence point in signaling pathways on cell proliferation and apoptosis. This article first reviews the unique function of p66Shc protein in regulating oxidative stress-induced apoptosis. Subsequently, we discuss its novel role in androgen-regulated prostate cancer cell proliferation and metastasis and the mechanism by which it mediates androgen action via the redox signaling pathway. The data together indicate that p66Shc might be a useful biomarker for the prognosis of prostate cancer and serve as an effective target for its cancer treatment.




Similar content being viewed by others
References
Guo, M., & Hay, B. A. (1999). Cell proliferation and apoptosis. Current Opinion in Cell Biology, 11, 745–752.
Evan, G. I., & Vousden, K. H. (2001). Proliferation, cell cycle and apoptosis in cancer. Nature, 411, 342–348.
Rathmell, J. C., & Thompson, C. B. (1999). The central effectors of cell death in the immune system. Annual Review of Immunology, 17, 781–828.
Ginaldi, L., De Martinis, M., D'Ostilio, A., Marini, L., Loreto, M. F., Corsi, M. P., et al. (2000). Cell proliferation and apoptosis in the immune system in the elderly. Immunological Research, 21, 31–38.
Gross, A., McDonnell, J. M., & Korsmeyer, S. J. (1999). BCL-2 family members and the mitochondria in apoptosis. Genes and Development, 13, 1899–1911.
Budd, R. C. (2002). Death receptors couple to both cell proliferation and apoptosis. Journal of Clinical Investigation, 109, 437–441.
Pelicci, G., Lanfrancone, L., Grignani, F., McGlade, J., Cavallo, F., Forni, G., et al. (1992). A novel transforming protein [SHC] with an SH2 domain is implicated in mitogenic signal transduction. Cell, 70, 93–104.
Migliaccio, E., Mele, S., Salcini, A. E., Pelicci, G., Lai, K. M., Superti-Furga, G., et al. (1997). Opposite effects of the p52Shc/p46Shc and p66Shc splicing isoforms on the EGF receptor- MAP kinase-fos signaling pathway. The EMBO Journal, 16, 706–716.
Ravichandran, K. S. (2001). Signaling via Shc family adapter proteins. Oncogene, 20, 6322–6330.
Lee, M. S., Igawa, T., Chen, S. J., Van Bemmel, D., Lin, J. S., Lin, F. F., et al. (2004). p66Shc protein is upregulated by steroid hormones in hormone-sensitive cancer cells and in primary prostate carcinomas. International Journal of Cancer, 108, 672–678.
Migliaccio, E., Giorgio, M., & Pelicci, P. G. (2006). Apoptosis and aging: role of p66Shc redox protein. Antioxidant and Redox Signaling, 8, 600–608.
Henderson, B. E., & Feigelson, H. S. (2000). Hormonal carcinogenesis. Carcinogenesis, 21, 427–433.
Meng, T. C., Lee, M. S., & Lin, M. F. (2000). Interaction between protein tyrosine phosphatase and protein tyrosine kinase is involved in androgen-promoted growth of human prostate cancer cells. Oncogene, 19, 2664–2677.
Grossmann, M. E., Huang, H., & Tindall, D. J. (2001). Androgen receptor signaling in androgen-refractory prostate cancer. Journal of the National Cancer Institute, 93, 1687–1697.
Kraus, S., Gioeli, D., Vomastek, T., Gordon, V., & Weber, M. J. (2006). Receptor for activated C kinase 1 [RACK1] and Src regulate the tyrosine phosphorylation and function of the androgen receptor. Cancer Research, 66, 11047–11054.
Guo, Z., Dai, B., Jiang, T., Xu, K., Xie, Y., & Kim, O. (2006). Regulation of androgen receptor activity by tyrosine Phosphorylation. Cancer Cell, 10, 309–319.
Weigel, N. L., & Moore, N. L. (2007). Kinases and protein phosphorylation as regulators of steroid hormone action. Nuclear Receptor Signaling, 5, e005.
Dickson, R. B., & Lippman, M. E. (1987). Estrogenic regulation of growth and polypeptide growth factor secretion in human breast carcinoma. Endocrine Reviews, 8, 29–43.
Dabrosin, C., Ollinger, K., Ungerstedt, U., & Hammar, M. (1997). Variability of glutathione levels in normal breast tissue and subcutaneous fat during the menstrual cycle: an in vivo study with microdialysis technique. Journal of Clinical Endocrinology and Metabolism, 82, 1382–1384.
Devanesan, P., Santen, R. J., Bocchinfuso, W. P., Korach, K. S., Rogan, E. G., & Cavalieri, E. (2001). Catechol estrogen metabolites and conjugates in mammary tumors and hyperplastic tissue from estrogen receptor-alpha knock-out [ERKO]/Wnt-1 mice: Implications for initiation of mammary tumors. Carcinogenesis, 22, 1573–1576.
Engebraaten, O., Bjerkvig, R., Pedersen, P. H., & Laerum, O. D. (1993). Effects of EGF, bFGF, NGF, and PDGF [bb] on cell proliferative, migratory, and invasive capacities of human brain-tumour biopsies in vitro. International Journal of Cancer, 53, 209–214.
Hamada, J., Nagayasu, H., Takayama, M., Kawano, T., Hosokawa, M., & Takeichi, N. (1995). Enhanced effect of epidermal growth factor on pulmonary metastasis and in vitro invasion of rat mammary carcinoma cells. Cancer Letters, 89, 161–167.
Mignatti, P., Morimoto, T., & Rifkin, D. B. (1991). Basic fibroblast growth factor released by single, isolated cells stimulates their migration in an autocrine manner. Proceedings of National Academy of Sciences, 88, 11007–11011.
Taylor, W. R., Greenberg, A. H., Turley, E. A., & Wright, J. A. (1993). Cell motility, invasion, and malignancy induced by overexpression of K-FGF or bFGF. Experimental cell research, 204, 295–301.
Choudhury, G. G., Karamitsos, C., Hernandez, J., Gentilini, A., Bardgette, J., & Abboud, H. E. (1997). PI-3-kinase and MAPK regulate mesangial cell proliferation and migration in response to PDGF. Amarican Journal of Physiology, 273, F931–F938.
Stracke, M. L., Engel, J. D., Wilson, L. W., Rechler, M. M., Liotta, L. A., & Schiffmann, E. (1989). The type I insulin-like growth factor receptor is a motility receptor in human melanoma cells. Journal of Biological Chemistry, 264, 21544–21549.
Sachs, M., Weidner, K. M., Brinkmann, V., Walther, I., Obermeier, A., Ullrich, A., et al. (1996). Motogenic and morphogenic activity of epithelial receptor tyrosine kinases. The Journal of Cell Biology, 133, 1095–1107.
Pelicci, G., Giordano, S., Zhen, Z., Salcini, A. E., Lanfrancone, L., Bardelli, A., et al. (1995). The motogenic and mitogenic responses to HGF are amplified by the Shc adaptor protein. Oncogene, 10, 1631–1638.
Ratner, S., Patrick, P., & Bora, G. (1992). Lymphocyte development of adherence and motility in extracellular matrix during IL-2 stimulation. The Journal of Immunology, 149, 681–688.
Migliaccio, E., Giorgio, M., Mele, S., Pelicci, G., Reboldi, P., Pandolfi, P. P., et al. (1999). The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature, 402, 309–313.
Orr, W. C., & Sohal, R. S. (1994). Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science, 263, 1128–1130.
Taub, J., Lau, J. F., Ma, C., Hahn, J. H., Hoque, R., Rothblatt, J., et al. (1999). A cytosolic catalase is needed to extend adult lifespan in C. elegans daf-C and clk-1 mutants. Nature, 399, 162–166.
Sagi, O., Wolfson, M., Utko, N., Muradian, K., & Fraifeld, V. (2005). p66ShcA and ageing: modulation by longevity-promoting agent aurintricarboxylic acid. Mechanisms of Ageing and Development, 126, 249–254.
Jackson, J. G., Yoneda, T., Clark, G. M., & Yee, D. (2000). Elevated levels of p66 Shc are found in breast cancer cell lines and primary tumors with high metastatic potential. Clinical Cancer Research, 6, 1135–1139.
Luzi, L., Confalonieri, S., Di Fiore, P. P., & Pelicci, P. G. (2000). Evolution of Shc functions from nematode to human. Current Opinion in Genetics and Development, 10, 668–674.
Davol, P. A., Bagdasaryan, R., Elfenbein, G. J., Maizel, A. L., & Frackelton, A. R., Jr. (2003). Shc proteins are strong, independent prognostic markers for both node negative and node positive primary breast cancer. Cancer Research, 63, 6772–6783.
De, S., Razorenova, O., McCabe, N. P., O'Toole, T., Qin, J., & Byzova, T. V. (2005). VEGF-integrin interplay controls tumor growth and vascularization. Proceedings of the National Academy of Sciences, 102, 7589–7594.
Grossman, S. R., Lyle, S., Resnick, M. B., Sabo, E., Lis, R. T., Rosinha, E., et al. (2007). p66 Shc tumor levels show a strong prognostic correlation with disease outcome in stage IIA colon cancer. Clinical Cancer Research, 13, 5798–5804.
Biscardi, J. S., Belsches, A. P., & Parsons, S. J. (1998). Characterization of human epidermal growth factor receptor and c-Src interactions in human breast tumor cells. Molecular Carcinoenesis, 21, 261–272.
Stevenson, L. E., & Frackelton, A. R., Jr. (1998). Constitutively tyrosine phosphorylated p52 Shc in breast cancer cells: correlation with ErbB2 and p66 Shc expression. Breast Cancer Research and Treatment, 49, 119–128.
Veeramani, S., Igawa, T., Yuan, T. C., Lin, F. F., Lee, M. S., Lin, J. S., et al. (2005). Expression of p66 [Shc] protein correlates with proliferation of human prostate cancer cells. Oncogene, 24, 7203–7212.
Veeramani, S., Yuan, T. C., Lin, F. F., & Lin, M. F. (2008). Mitochondrial redox signaling by p66 Shc is involved in regulating androgenic growth stimulation of human prostate cancer cells. Oncogene, 27, 5057–5068.
Ventura, A., Luzi, L., Pacini, S., Baldari, C. T., & Pelicci, P. G. (2002). The p66 Shc longevity gene is silenced through epigenetic modifications of an alternative promoter. Journal of Biological Chemistry, 277, 22370–22376.
Pawson, T., & Scott, J. D. (1997). Signaling through scaffold, anchoring, and adaptor proteins. Science, 278, 2075–2080.
Yoshida, S., Masaki, T., Feng, H., Yuji, J., Miyauchi, Y., Funaki, T., et al. (2004). Enhanced expression of adaptor molecule p46 Shc in nuclei of hepatocellular carcinoma cells: study of LEC rats. International Journal of Oncology, 25, 1089–1096.
Giorgio, M., Migliaccio, E., Orsini, F., Paolucci, D., Moroni, M., Contursi, C., et al. (2005). Electron transfer between cytochrome c and p66 Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell, 122, 221–233.
Pellegrini, M., Pacini, S., & Baldari, C. T. (2005). p66SHC: the apoptotic side of Shc proteins. Apoptosis, 10, 13–18.
Xie, Y., & Hung, M. C. (1996). p66Shc isoform down-regulated and not required for HER-2/neu signaling pathway in human breast cancer cell lines with HER-2/neu overexpression. Biochemical and Biophysical Research Communications, 221, 140–145.
Pacini, S., Pellegrini, M., Migliaccio, E., Patrussi, L., Ulivieri, C., Ventura, A., et al. (2004). p66SHC promotes apoptosis and antagonizes mitogenic signaling in T cells. Molecular Cell Biology, 24, 1747–1757.
Le, S., Connors, T. J., & Maroney, A. C. (2001). c-Jun N-terminal kinase specifically phosphorylates p66ShcA at serine 36 in response to ultraviolet irradiation. Journal of Biological Chemistry, 276, 48332–48336.
Kao, A. W., Waters, S. B., Okada, S., & Pessin, J. E. (1997). Insulin stimulates the phosphorylation of the 66- and 52-kilodalton Shc isoforms by distinct pathways. Endocrinology, 138, 2474–2480.
Yang, C. P., & Horwitz, S. B. (2000). Taxol mediates serine phosphorylation of the 66-kDa Shc isoform. Cancer Research, 60, 5171–5178.
Okada, N., Wada, K., Goldsmith, B. A., & Koizumi, S. (1997). The 66-kDa Shc isoform is a negative regulator of the epidermal growth factor-stimulated mitogen- activated protein kinase pathway. Journal of Biological Chemistry, 272, 28042–28049.
Khanday, F. A., Yamamori, T., Mattagajasingh, I., Zhang, Z., Bugayenko, A., Naqvi, A., et al. (2006). Rac1 leads to phosphorylation-dependent increase in stability of the p66shc adaptor protein: role in Rac1-induced oxidative stress. Molecular Biology of the Cell, 17, 122–129.
Pinton, P., Rimessi, A., Marchi, S., Orsini, F., Migliaccio, E., Giorgio, M., et al. (2007). Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science, 315, 659–663.
Geisel, J., Schorr, H., Heine, G. H., Bodis, M., Hübner, U., Knapp, J. P., et al. (2007). Decreased p66Shc promoter methylation in patients with end-stage renal disease. Clinical Chemistry Laboratory Medicine, 45, 1764–1770.
Richardson, B. C. (2002). Role of DNA methylation in the regulation of cell function: autoimmunity, aging and cancer. The Journal of Nutrition, 132, 2401S–2405S.
Baylin, S. B., Herman, J. G., Graff, J. R., Vertino, P. M., & Issa, J. P. (1998). Alterations in DNA methylation: a fundamental aspect of neoplasia. Advances in Cancer Research., 72, 141–196.
Ahuja, N., & Issa, J. P. (2000). Aging, methylation and cancer. Histology and Histopathology, 15, 835–842.
Ahuja, N., Li, Q., Mohan, A. L., Baylin, S. B., & Issa, J. P. (1998). Aging and DNA methylation in colorectal mucosa and cancer. Cancer Research, 58, 5489–5494.
Issa, J. P., Ottaviano, Y. L., Celano, P., Hamilton, S. R., Davidson, N. E., & Baylin, S. B. (1994). Methylation of the oestrogen receptor CpG island links ageing and neoplasia in human colon. Nature Genetics, 7, 536–540.
Pandolfi, S., Bonafè, M., Di Tella, L., Tiberi, L., Salvioli, S., Monti, D., et al. (2005). p66[shc] is highly expressed in fibroblasts from centenarians. Mechanism of Ageing and Development, 126, 839–844.
Mooijaart, S. P., van Heemst, D., Schreuder, J., van Gerwen, S., Beekman, M., Brandt, B. W., et al. (2004). Variation in the SHC1 gene and longevity in humans. Experimental Gerontology, 39, 263–268.
Natalicchio, A., Laviola, L., De Tullio, C., Renna, L. A., Montrone, C., Perrini, S., et al. (2004). Role of the p66Shc isoform in insulin-like growth factor I receptor signaling through MEK/Erk and regulation of actin cytoskeleton in rat myoblasts. Journal of Biological Chemistry, 279, 43900–43909.
Foschi, M., Franchi, F., Han, J., La Villa, G., & Sorokin, A. (2001). Endothelin-1 induces serine phosphorylation of the adaptor protein p66Shc and its association with 14-3-3 protein in glomerular mesangial cells. Journal of Biological Chemistry, 276, 26640–26647.
Faisal, A., el-Shemerly, M., Hess, D., & Nagamine, Y. (2002). Serine/threonine phosphorylation of ShcA. Regulation of protein-tyrosine phosphatase-pest binding and involvement in insulin signaling. Journal of Biological Chemistry, 277, 30144–30152.
Yang, C. P., & Horwitz, S. B. (2002). Distinct mechanisms of taxol-induced serine phosphorylation of the 66-kDa Shc isoform in A549 and RAW 264.7 cells. Biochimica et Biophysica Acta, 1590, 76–83.
Arany, I., Faisal, A., Nagamine, Y., & Safirstein, R. L. (2008). p66shc inhibits pro-survival epidermal growth factor receptor/ERK signaling during severe oxidative stress in mouse renal proximal tubule cells. Journal of Biological Chemistry, 283, 6110–6117.
El-Shemerly, M. Y., Besser, D., Nagasawa, M., & Nagamine, Y. (1997). 12-O- Tetradecanoylphorbol-13-acetate activates the Ras/extracellular signal-regulated kinase [ERK] signaling pathway upstream of SOS involving serine phosphorylation of Shc in NIH3T3 cells. Journal of Biological Chemistry, 272, 30599–30602.
Holt, K. H., Kasson, B. G., & Pessin, J. E. (1996). Insulin stimulation of a MEK- dependent but ERK-independent SOS protein kinase. Molecular and Cellular Biology, 16, 577–583.
Purdom, S., & Chen, Q. M. (2003). p66[Shc]: at the crossroad of oxidative stress and the genetics of aging. Trends in Molecular Medicine, 9, 206–210.
Chao, D. T., & Korsmeyer, S. J. (1998). BCL-2 family: regulators of cell death. Annual Review of Immunology, 16, 395–419.
Shichiri, M., Yokokura, M., Marumo, F., & Hirata, Y. (2000). Endothelin-1 inhibits apoptosis of vascular smooth muscle cells induced by nitric oxide and serum deprivation via MAP kinase pathway. Arteriosclerosis, Thrombosis, and Vascular Biology, 20, 989–997.
Habib, T., Herrera, R., & Decker, S. J. (1994). Activators of protein kinase C stimulate association of Shc and the PEST tyrosine phosphatase. Journal of Biological Chemistry, 269, 25243–25246.
Charest, A., Wagner, J., Jacob, S., McGlade, C. J., & Tremblay, M. L. (1996). Phosphotyrosine-independent binding of SHC to the NPLH sequence of murine protein-tyrosine phosphatase-PEST. Evidence for extended phosphotyrosine binding/phosphotyrosine interaction domain recognition specificity. Journal of Biological Chemistry, 271, 8424–8429.
Nemoto, S., & Finkel, T. (2002). Redox regulation of forkhead proteins through a p66shc-depedent signaling pathway. Science, 295, 2450–2452.
Trinei, M., Giorgio, M., Cicalese, A., Barozzi, S., Ventura, A., Migliaccio, E., et al. (2002). A p53-p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene, 21, 3872–3878.
Napoli, C., Martin-Padura, I., de Nigris, F., Giorgio, M., Mansueto, G., Somma, P., et al. (2003). Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proceedings of the National Academy of Sciences of the United States of America, 100, 2112–2116.
Francia, P., delli Gatti, C., Bachschmid, M., Martin-Padura, I., Savoia, C., Migliaccio, E., et al. (2004). Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation, 110, 2889–2895.
Zaccagnini, G., Martelli, F., Fasanaro, P., Magenta, A., Gaetano, C., Di Carlo, A., et al. (2004). p66ShcA modulates tissue response to hindlimb ischemia. Circulation, 109, 2917–2923.
Graiani, G., Lagrasta, C., Migliaccio, E., Spillmann, F., Meloni, M., Madeddu, P., et al. (2005). Genetic deletion of the p66Shc adaptor protein protects from angiotensin II-induced myocardial damage. Hypertension, 46, 433–440.
Koch, O. R., Fusco, S., Ranieri, S. C., Maulucci, G., Palozza, P., Larocca, L. M., et al. (2008). Role of the life span determinant P66[shcA] in ethanol-induced liver damage. Laboratory Investigations, 88, 750–760.
Sen, C. K., & Packer, L. (1996). Antioxidant and redox regulation of gene transcription. The FASEB Journal, 10, 709–720.
Orsini, F., Migliaccio, E., Moroni, M., Contursi, C., Raker, V. A., Piccini, D., et al. (2004). The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. Journal of Biological Chemistry, 279, 25689–25695.
Smith, W. W., Norton, D. D., Gorospe, M., Jiang, H., Nemoto, S., Holbrook, N. J., et al. (2005). Phosphorylation of p66Shc and forkhead proteins mediates Aβ toxicity. The Journal of Cell Biology, 169, 331–339.
Stevenson, M. A., Pollock, S. S., Coleman, C. N., & Calderwood, S. K. (1994). X- irradiation, phorbol esters, and H2O2 stimulate mitogen-activated protein kinase activity in NIH-3T3 cells through the formation of reactive oxygen intermediates. Cancer Research, 54, 12–15.
Polyak, K., Xia, Y., Zweier, J. L., Kinzler, K. W., & Vogelstein, B. (1997). A model for p53-induced apoptosis. Nature, 389, 300–305.
Li, P. F., Dietz, R., & von Harsdorf, R. (1999). p53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by Bcl-2. The EMBO Journal, 18, 6027–6036.
Schuler, M., Bossy-Wetzel, E., Goldstein, J. C., Fitzgerald, P., & Green, D. R. (2000). p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. Journal of Biological Chemistry, 275, 7337–7342.
Tiberi, L., Faisal, A., Rossi, M., Di Tella, L., Franceschi, C., & Salvioli, S. (2006). p66[Shc] gene has a pro-apoptotic role in human cell lines and it is activated by a p53-independent pathway. Biochemical and Biophysical Research Communications, 342, 503–508.
Favetta, L. A., St. John, E. J., King, W. A., & Betts, D. H. (2007). High levels of p66shc and intracellular ROS in permanently arrested early embryos. Free Radical Biology and Medicine, 42, 1201–1210.
Favetta, L. A., Robert, C., St. John, E. J., Betts, D. H., & King, W. A. (2004). p66shc, but not p53, is involved in early arrest of in vitro-produced bovine embryos. Molecular Human Reproduction, 10, 383–392.
Matwee, C., Betts, D. H., & King, W. A. (2000). Apoptosis in the early bovine embryo. Zygote, 8, 57–68.
Velez-Pardo, C., Morales, A. T., Del Rio, M. J., & Olivera-Angel, M. (2007). Endogenously generated hydrogen peroxide induces apoptosis via mitochondrial damage independent of NF-kappaB and p53 activation in bovine embryos. Theriogenology, 67, 1285–1296.
Betts, D. H., & Madan, P. (2008). Permanent embryo arrest: molecular and cellular concepts. Molecular Human Reproduction, 14, 445–453.
Tang, E. D., Nuñez, G., Barr, F. G., & Guan, K. L. (1999). Negative regulation of the forkhead transcription factor FKHR by Akt. Journal of Biological Chemistry, 274, 16741–16746.
Biggs, W. H., 3rd, Cavenee, W. K., & Arden, K. C. (2001). Identification and characterization of members of the FKHR [FOX O] subclass of winged-helix transcription factors in the mouse. Mammalian Genome, 12, 416–425.
Brunet, A., Bonni, A., Zigmond, M. J., Lin, M. Z., Juo, P., Hu, L. S., et al. (1999). Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell, 96, 857–868.
Kops, G. J., Dansen, T. B., Polderman, P. E., Saarloos, I., Wirtz, K. W., Coffer, P. J., et al. (2002). Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature, 419, 316–321.
Dijkers, P. F., Medema, R. H., Lammers, J. W., Koenderman, L., & Coffer, P. J. (2000). Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Current Biology, 10, 1201–1204.
Ventura, A., Maccarana, M., Raker, V. A., & Pelicci, P. G. (2004). A cryptic targeting signal induces isoform-specific localization of p46Shc to mitochondria. Journal of Biological Chemistry, 279, 2299–2306.
Nemoto, S., Combs, C. A., French, S., Ahn, B. H., Fergusson, M. M., Balaban, R. S., et al. (2006). The mammalian longevity-associated gene product p66shc regulates mitochondrial metabolism. Journal of Biological Chemistry, 281, 10555–10560.
Rizzuto, R., Pinton, P., Carrington, W., Fay, F. S., Fogarty, K. E., Lifshitz, L. M., et al. (1998). Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science, 280, 1763–1766.
Pinton, P., Leo, S., Wieckowski, M. R., Di Benedetto, G., & Rizzuto, R. (2004). Long-term modulation of mitochondrial Ca2+ signals by protein kinase C isozymes. The Journal of Cell Biology, 165, 223–232.
Gopalakrishna, R., & Jaken, S. (2000). Protein kinase C signaling and oxidative stress. Free Radical Biology of Medicine, 28, 1349–1361.
Pellegrini, M., Finetti, F., Petronilli, V., Ulivieri, C., Giusti, F., Lupetti, P., et al. (2007). p66SHC promotes T cell apoptosis by inducing mitochondrial dysfunction and impaired Ca2+ homeostasis. Cell Death and Differentiation, 14, 338–347.
Wulf, G., Finn, G., Suizu, F., & Lu, K. P. (2005). Phosphorylation-specific prolyl isomerization: is there an underlying theme? Nature Cell Biology, 7, 435–441.
Orsini, F., Moroni, M., Contursi, C., Yano, M., Pelicci, P., Giorgio, M., et al. (2006). Regulatory effects of the mitochondrial energetic status on mitochondrial p66Shc. Biological Chemistry, 387, 1405–1410.
Gertz, M., Fischer, F., Wolters, D., & Steegborn, C. (2008). Activation of the lifespan regulator p66Shc through reversible disulfide bond formation. Proceedings of the National Academic of Sciences of the United States of America, 105, 5705–5709.
Sastre, J., Pallardó, F. V., & Viña, J. (2003). The role of mitochondrial oxidative stress in aging. Free Radical Biology of Medicine, 35, 1–8.
Ames, B. N., Shigenaga, M. K., & Hagen, T. M. (1993). Oxidants, antioxidants, and the degenerative diseases of aging. Proceedings of the National Academy of Sciences of the United States of America, 90, 7915–7922.
Lithgow, G. J., & Kirkwood, T. B. (1996). Mechanisms and evolution of aging. Science, 273, 80.
Helbock, H. J., Beckman, K. B., & Ames, B. N. (1999). 8-Hydroxydeoxyguanosine and 8-hydroxyguanine as biomarkers of oxidative DNA damage. Methods in Enzymology, 300, 156–166.
Favetta, L. A., Robert, C., King, W. A., & Betts, D. H. (2004). Expression profiles of p53 and p66shc during oxidative stress-induced senescence in fetal bovine fibroblasts. Experimental Cell Research, 299, 36–48.
Jiang, X., Edstrom, E., Altun, M., & Ulfhake, B. (2003). Differential regulation of Shc adaptor proteins in skeletal muscle, spinal cord and forebrain of aged rats with sensorimotor impairment. Aging Cell, 2, 47–57.
Klein, L. E., Freeze, B. S., Smith, A. B., 3rd, & Horwitz, S. B. (2005). The microtubule stabilizing agent discodermolide is a potent inducer of accelerated cell senescence. Cell Cycle, 4, 501–507.
Trinei, M., Berniakovich, I., Pelicci, P. G., & Giorgio, M. (2006). Mitochondrial DNA copy number is regulated by cellular proliferation: a role for Ras and p66[Shc]. Biochim Biophysics Acta, 1757, 624–630.
Masuyama, M., Iida, R., Takatsuka, H., Yasuda, T., & Matsuki, T. (2005). The pregnane X receptor regulates gene expression in a ligand- and promoter-selective fashion. Molecular Endocrinology, 19, 1170–1180.
Pages, G., Lenormand, P., L'Allemain, G., Chambard, J. C., Meloche, S., & Pouyssegur, J. (1993). Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proceedings of National Academy of Sciences, 90, 8319–8323.
Olson, M. F., Ashworth, A., & Hall, A. (1995). An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science, 269, 1270–1272.
Anand-Apte, B., Zetter, B. R., Viswanathan, A., Qiu, R. G., Chen, J., Ruggieri, R., et al. (1997). Platelet-derived growth factor and fibronectin-stimulated migration are differentially regulated by the Rac and extracellular signal-regulated kinase pathways. Journal of Biological Chemistry, 272, 30688–30692.
Klemke, R. L., Cai, S., Giannini, A. L., Gallagher, P. J., de Lanerolle, P., & Cheresh, D. A. (1997). Regulation of cell motility by mitogen-activated protein kinase. The Journal of Cell Biology, 137, 481–492.
Klemke, R. L., Yebra, M., Bayna, E. M., & Cheresh, D. A. (1994). Receptor tyrosine kinase signaling required for integrin alpha v beta 5-directed cell motility but not adhesion on vitronectin. The Journal of Cell Biology, 127, 859–866.
Huttenlocher, A., Sandborg, R. R., & Horwitz, A. F. (1995). Adhesion in cell migration. Current Opinion in Cell Biology, 7, 697–706.
Lauffenburger, D. A., & Horwitz, A. F. (1996). Cell migration: a physically integrated molecular process. Cell, 84, 359–369.
Abdollahi, A., Gruver, B. N., Patriotis, C., & Hamilton, T. C. (2003). Identification of epidermal growth factor-responsive genes in normal rat ovarian surface epithelial cells. Biochemical and Biophysical Research Communications, 18, 188–197.
Park, Y. J., Kim, T. Y., Lee, S. H., Kim, H., Kim, S. W., Shong, M., et al. (2005). p66shc expression in proliferating thyroid cells is regulated by thyrotropin receptor signaling. Endocrinology, 146, 2473–2480.
Ruoslahti, E., & Reed, J. C. (1994). Anchorage dependence, integrins, and apoptosis. Cell, 77, 477–478.
Hynes, R. O., Bader, B. L., & Hodivala-Dilke, K. (1999). Integrins in vascular development. Brazil Journal of Medicinal Biological Research, 32, 501–510.
Cooper, C. R., Chay, C. H., & Pienta, K. J. (2002). The role of alpha[v]beta[3] in prostate cancer progression. Neoplasia, 4, 191–194.
Zheng, D. Q., Woodard, A. S., Fornaro, M., Tallini, G., & Languino, L. R. (1999). Prostatic carcinoma cell migration via alpha[v]beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Research, 59, 1655–1664.
Northey, J. J., Chmielecki, J., Ngan, E., Russo, C., Annis, M. G., Muller, W. J., et al. (2008). Signaling through ShcA is required for transforming growth factor beta- and Neu/ErbB-2-induced breast cancer cell motility and invasion. Molecular and Cellular Biology, 28, 3162–3176.
Ursini-Siegel, J., & Muller, W. J. (2008). The ShcA adaptor protein is a critical regulator of breast cancer progression. Cell Cycle, 7, 1936–1943.
Sundaresan, M., Yu, Z. X., Ferrans, V. J., Sulciner, D. J., Gutkind, J. S., Irani, K., et al. (1996). Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochemical Journal, 318, 379–382.
Liu, S. L., Lin, X., Shi, D. Y., Cheng, J., Wu, C. Q., & Zhang, Y. D. (2002). Reactive oxygen species stimulated human hepatoma cell proliferation via cross-talk between PI3-K/PKB and JNK signaling pathways. Archives of Biochemistry and Biophysics, 406, 173–182.
Finkel, T., & Holbrook, N. J. (2000). Oxidants, oxidative stress and the biology of ageing. Nature, 9, 239–247.
Lou, Y. W., Chen, Y. Y., Hsu, S. F., Chen, R. K., Lee, C. L., Khoo, K. H., et al. (2008). Redox regulation of the protein tyrosine phosphatase PTP1B in cancer cells. FEBS Journal, 275, 69–88.
Lin, M. F., & Meng, T. C. (1996). Tyrosine phosphorylation of a 185 kDa phosphoprotein [pp 185] inversely correlates with the cellular activity of human prostatic acid phosphatase. Biochemical and Biophysical Research Communications, 226, 206–213.
Lin, M. F., Lee, M. S., Zhou, X. W., Andressen, J. C., Meng, T. C., & Johansson, S. L. (2001). Decreased expression of cellular prostatic acid phosphatase increases umorigenicity of human prostate cancer cells. The Journal of Urology, 166, 1943–1950.
Veeramani, S., Yuan, T. C., Chen, S. J., Lin, F. F., Petersen, J. E., Shaheduzzaman, S., et al. (2005). Cellular prostatic acid phosphatase: a protein tyrosine phosphatase involved in androgen-independent proliferation of prostate cancer. Endocr-Related Cancer, 12, 805–822.
Lim, S. D., Sun, C., Lambeth, J. D., Marshall, F., Amin, M., Chung, L., et al. (2005). Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate, 62, 200–207.
Khanday, F. A., Santhanam, L., Kasuno, K., Yamamori, T., Naqvi, A., Dericco, J., et al. (2006). Sos-mediated activation of rac1 by p66shc. The Journal of Cell Biology, 172, 817–822.
Knight-Krajewski, S., Welsh, C. F., Liu, Y., Lyons, L. S., Faysal, J. M., Yang, E. S., et al. (2004). Deregulation of the Rho GTPase, Rac1, suppresses cyclin-dependent kinase inhibitor p21[CIP1] levels in androgen-independent human prostate cancer cells. Oncogene, 23, 5513–5522.
Veeramani, S., & Lin, M. F. (2007). Role of reactive oxygen species in carcinogenesis. In R. Banarjee (Ed.), Redox biochemistry (pp. 212–218). New Jersey: Wiley.
Lippman, S. M., Klein, E. A., Goodman, P. J., Lucia, M. S., Thompson, I. M., Ford, L. G., et al. (2009). Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). The Journal of the American Medical Association, 301, 39–51.
Sakai, H., Igawa, T., Saha, P. K., Nomata, K., Yushita, Y., Kanetake, H., et al. (1992). A case of prostatic carcinoma presenting as a metastatic orbital tumor. Hinyokika Kiyo. Acta Urologica Japonica [Kyoto], 38, 77–80.
Chu, T. M., & Lin, M. F. (1998). PSA and acid phosphatase in the diagnosis of prostate cancer. Journal of Clinical Ligand Assay, 21, 24–34.
Reif, A. E., Schlesinger, R. M., Fish, C. A., & Robinson, C. M. (1973). Acid phosphatase isozymes in cancer of the prostate. Cancer, 31, 689–699.
Foti, A. G., Cooper, J. F., Herschman, H., & Malvaez, R. R. (1977). Detection of prostatic cancer by solid-phase radioimmunoassay of serum prostatic acid phosphatase. New England Journal of Medicine, 297, 1357–1361.
Loor, R., Wang, M. C., Valenzuela, L., & Chu, T. M. (1981). Expression of prostatic acid phosphatase in human prostate cancer. Cancer Letters, 14, 63–69.
Pontes, J. E., Rose, N. R., Ercole, C., & Pierce, J. M., Jr. (1981). Immunofluorescence for prostatic acid phosphatase: clinical applications. The Journal of Urology, 126, 187–189.
Solin, T., Kontturi, M., Pohlmann, R., & Vihko, P. (1990). Gene expression and prostate specificity of human prostatic acid phosphatase [PAP]: evaluation by RNA blot analyses. Biochimica et Biophysica Acta, 1048, 72–77.
Sakai, H., Yogi, Y., Minami, Y., Yushita, Y., Kanetake, H., & Saito, Y. (1993). Prostate specific antigen and prostatic acid phosphatase immunoreactivity as prognostic indicators of advanced prostatic carcinoma. The Journal of Urology, 149, 1020–1023.
Gioeli, D., Mandell, J. W., Petroni, G. R., Frierson, H. F., Jr., & Weber, M. J. (1999). Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Research, 59, 279–284.
Price, D. T., Della Rocca, G., Guo, C., Ballo, M. S., Schwinn, D. A., & Luttrell, L. M. (1999). Activation of extracellular signal regulated kinase in human prostate cancer. The Journal of Urology, 162, 1537–1542.
Lee, M. S., Igawa, T., Yuan, T. C., Zhang, X. Q., Lin, F. F., & Lin, M. F. (2003). ErbB2 signaling is involved in regulating PSA secretion in androgen-independent human prostate cancer LNCaP C-81 cells. Oncogene, 22, 781–796.
Acknowledgement
This study was supported in part by National Cancer Institute, National Institutes of Health [R01 CA88184], Department of Defense [W81XWH-06-1-0070 and W81XWH-08-1-0459], Nebraska Research Initiative for Cancer Metastasis, and Nebraska Cancer and Smoking Disease Research Program LB 506 [2008-20 and 2010-18]. We thank Dr. Shouqiang Ouyang for reading, and Ms. Fen-Fen Lin for her tremendous contributions toward our studies on the functional role of p66Shc in carcinogenesis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajendran, M., Thomes, P., Zhang, L. et al. p66Shc—a longevity redox protein in human prostate cancer progression and metastasis. Cancer Metastasis Rev 29, 207–222 (2010). https://doi.org/10.1007/s10555-010-9213-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-010-9213-8