Summary
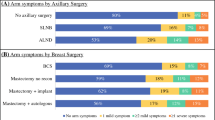
This study is the first large prospective RCT of sentinel node biopsy (SNB) compared with standard axillary treatment (level I-III axillary lymph node dissection or four node sampling), which includes comprehensive and repeated quality of life (QOL) assessments over 18 months. Patients (n=829) completed the Functional Assessment of Cancer Therapy – Breast (FACT-B+4) and the Spielberger State/Trait Anxiety Inventory (STAI) at baseline (pre-surgery) and at 1, 3, 6, 12, and 18 months post-surgery. There were significant differences between treatment groups favouring the SNB group throughout the 18 months assessment. Patients in the standard treatment group showed a greater decline in Trial Outcome Index (TOI) scores (physical well-being, functional well-being and breast cancer concerns subscales in FACT-B+4) and recovered more slowly than patients in the SNB group (p<0.01). The change in total FACT-B+4 scores (measuring global QOL) closely resembled the TOI results. 18 months post-surgery approximately twice as many patients in the standard group compared with the SNB group reported substantial arm swelling (14% versus 7%) (p=0.002) or numbness (19% versus 8.7%) (p<0.001). Despite the uncertainty about undergoing a relatively new procedure and the possible need for further surgery, there was no evidence of increased anxiety amongst patients randomised to SNB (p>0.05). For 6 months post-surgery younger patients reported less favourable QOL scores (p<0.001) and greater levels of anxiety (p<0.01). In view of the benefits regarding arm functioning and quality of life, the data from this randomised study support the use of SNB in patients with clinically node negative breast cancer.
Similar content being viewed by others
References
Mansel RE, Goyal A, European studies on breast lymphatic mappingSemin Oncol 31:304–310, 2004
Mansel RE, Goyal A, Fallowfield L, Newcombe RG: Sentinel node biopsy versus standard axillary treatment: results of the randomized multicenter UK ALMANAC trial. Abstracts of the 27th Annual San Antonio Breast Cancer Symposium. December 8–11, 2004, San Antonio, Texas, USA. Breast Cancer Res Treat 88(Suppl 1): S1–S265, 2004
Mansel RE, Goyal A, Fallowfield L, Newcombe RG: First results of the randomized multicenter ALMANAC trial. Abstracts of the 40th Annual Meeting of the American Society of Clinical Oncology. New Orleans, Louisiana, USA, June 5–8, 2004. J Clin Oncol 22: 1s–1069s, 2004
Schrenk P, Rieger R, Shamiyeh A, Wayand W, Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinomaCancer 88: 608–614, 2000
Temple LK, Baron R, Cody HS III, Fey JV, Thaler HT, Borgen PI, Heerdt AS, Montgomery LL, Petrek JA, Van Zee KJ, Sensory morbidity after sentinel lymph node biopsy and axillary dissection: a prospective study of 233 womenAnn Surg Oncol9: 654–662, 2002
Swenson KK, Nissen MJ, Ceronsky C, Swenson L, Lee MW, Tuttle TM, Comparison of side effects between sentinel lymph node and axillary lymph node dissection for breast cancerAnn Surg Oncol9: 745–753, 2002
Haid A, Koberle-Wuhrer R, Knauer M, Burtscher J, Fritzsche H, Peschina W, Jasarevic Z, Ammann M, Hergan K, Sturn H, et al. Morbidity of breast cancer patients following complete axillary dissection or sentinel node biopsy only: a comparative evaluationBreast Cancer Res Treat73: 31–36, 2002
Burak WE, Hollenbeck ST, Zervos EE, Hock KL, Kemp LC, Young DC, Sentinel lymph node biopsy results in less postoperative morbidity compared with axillary lymph node dissection for breast cancerAm J Surg183: 23–27, 2002
Baron RH, Fey JV, Borgen PI, Van Zee KJ, Eighteen sensations after breast cancer surgery: a two-year comparison of sentinel lymph node biopsy and axillary lymph node dissectionOncol Nurs Forum31: 691–698, 2004
Peintinger F, Reitsamer R, Stranzl H, Ralph G, Comparison of quality of life and arm complaints after axillary lymph node dissection vs. sentinel lymph node biopsy in breast cancer patientsBr J Cancer89: 648–652, 2003
Giuliano AE, Haigh PI, Brennan MB, Hansen NM, Kelley MC, Ye W, Glass EC, Turner RR, Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancerJ Clin Oncol18: 2553–2559, 2000
Kuehn T, Klauss W, Darsow M, Regele S, Flock F, Maiterth C, Dahlbender R, Wendt I, Kreienberg R, Long-term morbidity following axillary dissection in breast cancer patients-clinical assessment, significance for life quality and the impact of demographic, oncologic and therapeutic factorsBreast Cancer Res Treat64: 275–286, 2000
Haid A, Kuehn T, Konstantiniuk P, Koberle-Wuhrer R, Knauer M, Kreienberg R, Zimmermann G, Shoulder-arm morbidity following axillary dissection and sentinel node only biopsy for breast cancerEur J Surg Oncol28: 705–710, 2002
Arnaud S, Houvenaeghel G, Moutardier V, Butarelli M, Martino M, Tallet A, Braud AC, Jacquemier J, Julian-Reynier C, Brenot-Rossi I, Patients’ and surgeons’ perspectives on axillary surgery for breast cancerEur J Surg Oncol30: 735–743, 2004
Warmuth MA, Bowen G, Prosnitz LR, Chu L, Broadwater G, Peterson B, Leight G, Winer EP, Complications of axillary lymph node dissection for carcinoma of the breast: a report based on a patient surveyCancer83: 1362–1368, 1998
Blanchard DK, Donohue JH, Reynolds C, Grant CS: Relapse and morbidity in patients undergoing sentinel lymph node biopsy alone or with axillary dissection for breast cancer. Arch Surg 138: 482–487, discussion 487–488, 2003
Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, Intra M, Veronesi P, Robertson C, Maisonneuve P, et al.A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancerN Engl J Med349: 546–553, 2003
Rietman JS, Dijkstra PU, Geertzen JHB, Baas P, de Vries J, Dolsma WV, Groothoff JW, Eisma WH, Hoekstra HJ, Treatment-related upper limb morbidity 1 year after sentinel lymph node biopsy or axillary lymph node dissection for stage I or II breast cancerAnn Surg Oncol11: 1018–1024, 2004
Schijven MP, Vingerhoets AJ, Rutten HJ, Nieuwenhuijzen GA, Roumen RM, van Bussel ME, Voogd AC, Comparison of morbidity between axillary lymph node dissection and sentinel node biopsyEur J Surg Oncol29: 341–350, 2003
Ronka R, von Smitten K, Tasmuth T, Leidenius M, One-year morbidity after sentinel node biopsy and breast surgeryBreast14: 28–36, 2005
Yap KP, McCready DR, Narod S, Manchul LA, Trudeau M, Fyles A, Factors influencing arm and axillary symptoms after treatment for node negative breast carcinomaCancer97: 1369–1375, 2003
Liljegren G, Holmberg L, Arm morbidity after sector resection and axillary dissection with or without postoperative radiotherapy in breast cancer stage I. Results from a randomised trial. Uppsala-Orebro Breast Cancer Study GroupEur J Cancer33: 193–199, 1997
Hack TF, Cohen L, Katz J, Robson LS, Goss P, Physical and psychological morbidity after axillary lymph node dissection for breast cancerJ Clin Oncol17: 143–149, 1999
Ververs JM, Roumen RM, Vingerhoets AJ, Vreugdenhil G, Coebergh JW, Crommelin MA, Luiten EJ, Repelaer van Driel OJ, Schijven M, Wissing JC, et al. Risk, severity and predictors of physical and psychological morbidity after axillary lymph node dissection for breast cancerEur J Cancer37: 991–999, 2001
Engel J, Kerr J, Schlesinger-Raab A, Sauer H, Holzel D, Axilla surgery severely affects quality of life: results of a 5-year prospective study in breast cancer patientsBreast Cancer Res Treat79: 47–57, 2003
Dubernard G, Sideris L, Delaloge S, Marsiglia H, Rochard F, Travagli JP, Mathieu MC, Lumbroso J, Spielmann M, Garbay JR, et al. Quality of life after sentinel lymph node biopsy in early breast cancerEur J Surg Oncol30: 728–734, 2004
Keramopoulos A, Tsionou C, Minaretzis D, Michalas S, Aravantinos D, Arm morbidity following treatment of breast cancer with total axillary dissection: a multivariated approachOncology50: 445–449, 1993
Kiel KD, Rademacker AW, Early-stage breast cancer: arm edema after wide excision and breast irradiationRadiology198: 279–283, 1996
Coster S, Poole K, Fallowfield LJ, The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operativelyBreast Cancer Res Treat68: 273–282, 2001
Spielberger C, Gorsuch R, Lusherne R, Vagg P, Jacobs G: Manual for the State-trait Anxiety Inventory (form Y). Consulting Psychologists Press, Palo Alto, CA, 1983
Cella D, Tulsky D, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen S, Winicour P, Brannon J, The Functional Assessment of Cancer Therapy scale: development and validation of the general measureJ Clin Oncol11: 570–579, 1993
Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G, Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrumentJ Clin Oncol15: 974–986, 1997
Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A, Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trialJ Clin Oncol22: 4261–4271, 2004
Eton DT, Cella D, Yost KJ, Yount SE, Peterman AH, Neuberg DS, Sledge GW, Wood WC, A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scaleJ Clin Epidemiol57: 898–910, 2004
Cella D, Eton DT, Fairclough DL, Bonomi P, Heyes AE, Silberman C, Wolf MK, Johnson DH, What is a clinically meaningful change on the Functional Assessment of Cancer Therapy-Lung (FACT-L) questionnaire?: results from Eastern Cooperative Oncology Group (ECOG) study 5592J Clin Epidemiol55: 285–295, 2002
Passik SD, Newman ML, Brennan M, et al. Predictors of psychological distress, sexual dysfunction and physical functioning among women with upper extremity lymphedema related to breast cancerPsycho-Oncol4: 255–263, 1995
Voogd AC, Ververs JM, Vingerhoets AJ, Roumen RM, Coebergh JW, Crommelin MA, Lymphoedema and reduced shoulder function as indicators of quality of life after axillary lymph node dissection for invasive breast cancerBr J Surg90: 76–81, 2003
Edwards TL, Prevalence and aetiology of lymphoedema after breast cancer treatment in southern TasmaniaAust NZ J Surg70: 412–418, 2000
Poole K, Fallowfield LJ, The psychological impact of post-operative arm morbidity following axillary surgery for breast cancer: a critical reviewBreast11: 81–87, 2002
Maunsell E, Brisson J, Deschenes L, Arm problems and psychological distress after surgery for breast cancerCan J Surg36: 315–320, 1993
Fallowfield L, Offering choice of surgical treatment to women with breast cancer Patient Educ Couns30:209–214, 1997
Hatcher MB, Fallowfield L, A’Hern R, The psychosocial impact of bilateral prophylactic mastectomy: prospective study using questionnaires and semistructured InterviewsBMJ322: 76, 2001
Galper SR, Lee SJ, Tao ML, Troyan S, Kaelin CM, Harris JR, Weeks JC, Patient preferences for axillary dissection in the management of early-stage breast cancerJ Natl Cancer Inst92: 1681–1687, 2000
Asaad M, Tamer M, Mokbel K, British women’s choice between sentinel node biopsy and axillary node clearance for breast cancerCurr Med Res Opin19: 570–574, 2003
Gan S, Magarey C, Schwartz P, Papadatos G, Graham P, Vallentine J, Women’s choice between sentinel lymph node biopsy and axillary clearanceANZ J Surg72: 110–113, 2002
Acknowledgements
We thank all patients in the ALMANAC study, research fellows, other study investigators and all the surgery, nuclear medicine, radiological, radiographic, and nursing staff at each centre.
The validation phase of the study was supported by a grant from the UK Medical Research Council. The randomised phase was supported by grants from the Welsh Office of Research and Development, National Cancer Research Network, GE Healthcare, Wales Cancer Trials Network and Cancer Research UK funds (L. Fallowfield).
Steering committee: Robert Haward (Chairman, Cookridge Hospital, Leeds), David Cohen (University of Glamorgan, Treforest), Ian Ellis (Nottingham City Hospital, Nottingham), Emiel Rutgers (Netherlands Cancer Institute, Amsterdam), Robert Mansel-Principal Investigator (Cardiff University, Cardiff), Lesley Fallowfield (Cancer Research UK Psychosocial Oncology Group, Brighton); Ciaran Woodman (Christie Hospital, Manchester), Robert Newcombe (Cardiff University, Cardiff), Peter J Ell (The Middlesex Hospital, London).
Data monitoring committee: Mahesh Parmar (Chairman, Medical Research Council, London), John Northover (St Mark’s Hospital, London), John Yarnold (The Royal Marsden, London).
Trial coordinator: Julia Townson (Cardiff University, Cardiff).
Quality of Life: Karen Poole, Sam Coster, Rosemary Murray, Valerie Jenkins, (Cancer Research UK Psychosocial Oncology Group, Brighton).
Participating centres and investigators: Bangor, Gwynedd Hospitals NHS Trust-Derek Crawford. Birmingham, Queen Elizabeth Medical Centre-David England. Cardiff, Cardiff University-Robert Mansel, Ian Monypenny, Helen Sweetland, David Webster. Derby, Derby City General Hospital-Mark Sibbering, Howard Holliday. Edinburgh, Western General Hospital-Utheshtra Chetty, J. Michael Dixon. Guildford, Royal Surrey County Hospital-Mark Kissin. Huddersfield, The Royal Infirmary-Richard Sainsbury. Hull, Castle Hill Hospital-Tapan Mahapatra. Leeds, Leeds General Infirmary-Kieran Horgan. London, Charing Cross Hospital-Dudley H. Sinnett. Manchester, South Manchester University Hospital-Lester Barr, Nigel Bundred. Nottingham, Nottingham City Hospital-James Geraghty. Portsmouth, Queen Alexandra Hospital-Constantinos Yiangou. Rhyl, Glan Clwyd Hospital-Christopher Davies. Swansea, Morriston Hospital-Mike Chare. Peterborough, Edith Cavell Hospital-Tholkifl Abdullah.
Author information
Authors and Affiliations
Corresponding author
Additional information
Address for offprints and correspondence: Professor Lesley Fallowfield, Cancer Research UK Psychosocial Oncology Group, Brighton & Sussex Medical School, University of Sussex, Falmer, Brighton BN1 9QG, United Kingdom; Tel.: +44-0-1273-873015; Fax: +44-0-1273-873022; E-mail: l.j.fallowfield@sussex.ac.uk
Rights and permissions
About this article
Cite this article
Fleissig, A., Fallowfield, L.J., Langridge, C.I. et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat 95, 279–293 (2006). https://doi.org/10.1007/s10549-005-9025-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-005-9025-7