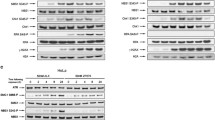
Abstract
The topoisomerase IIα inhibitor etoposide is a ‘broad spectrum’ anticancer agent and a potent inducer of DNA double strand breaks. DNA damage response of mammalian cells usually involves cell cycle arrest and DNA repair or, if unsuccessful, cell death. We investigated these processes in the human colon cancer cell line HT-29 treated with three different etoposide regimens mimicking clinically relevant plasma concentrations of cancer patients. Each involved a period of drug-free incubation following etoposide exposure to imitate the decline of plasma levels between the cycles of chemotherapy. We found a massive induction of double strand breaks that were rapidly and nearly completely fixed long before the majority of cells underwent apoptosis or necrosis. An even greater percentage of cells lost clonogenicity. The occurrence of double strand breaks was accompanied by a decrease in the levels of Ku70, Ku86 and DNA-PKcs as well as an increase in the level of Rad51 protein. Twenty-four hours after the first contact with etoposide we found a pronounced G2/M arrest, regardless of the duration of drug exposure, the level of double strand breaks and the extent of their repair. During the subsequent drug-free incubation period, the loss of clonogenicity correlated well with the preceding G2/M arrest as well as with the amount of cell death found several days after exposure. However, it correlated neither with early apoptosis or necrosis nor with any of the other investigated parameters. These results suggest that the G2/M arrest is an important determinant in the cytostatic action of etoposide and that the removal of DNA double strand breaks is not sufficient to ensure cell survival.








Similar content being viewed by others
References
Walker JV, Nitiss JL (2002) DNA topoisomerase II as a target for cancer chemotherapy. Cancer Invest 20:570–589
Gieseler F, Bauer E, Nuessler V, Clark M, Valsamas S (1999) Molecular effects of topoisomerase II inhibitors in AML cell lines: correlation of apoptosis with topoisomerase II activity but not with DNA damage. Leukemia 13:1859–1863
Wang Y, Zhou R, Liliemark J, Gruber A, Lindemalm S, Albertioni F, Liliemark E (2001) In vitro topo II–DNA complex accumulation and cytotoxicity of etoposide in leukaemic cells from patients with acute myelogenous and chronic lymphocytic leukaemia. Leuk Res 25:133–140. doi:10.1016/S0145-2126(00)00103-X
Baguley BC, Ferguson LR (1998) Mutagenic properties of topoisomerase-targeted drugs. Biochim Biophys Acta 1400:213–222. doi:10.1016/S0167-4781(98)00137-7
Felix CA (2001) Leukemias related to treatment with DNA topoisomerase II inhibitors. Med Pediatr Oncol 36:525–535
Felix CA, Kolaris CP, Osheroff N (2006) Topoisomerase II and the etiology of chromosomal translocations. DNA Repair (Amst) 5:1093–1108. doi:10.1016/j.dnarep.2006.05.031
Mistry AR, Felix CA, Whitmarsh RJ, Mason A, Reiter A, Cassinat B, Parry A, Walz C, Wiemels JL, Segal MR, Ades L, Blair IA, Osheroff N, Peniket AJ, Lafage-Pochitaloff M, Cross NC, Chomienne C, Solomon E, Fenaux P, Grimwade D (2005) DNA topoisomerase II in therapy-related acute promyelocytic leukemia. N Engl J Med 352:1529–1538
Ohshima A, Miura I, Chubachi A, Hashimoto K, Nimura T, Utsumi S, Takahashi N, Hayashi Y, Seto M, Ueda R, Miura AB (1996) 11q23 aberration is an additional chromosomal change in de novo acute leukemia after treatment with etoposide and mitoxantrone. Am J Hematol 53:264–266
Sandoval C, Pui CH, Bowman LC, Heaton D, Hurwitz CA, Raimondi SC, Behm FG, Head DR (1993) Secondary acute myeloid leukemia in children previously treated with alkylating agents, intercalating topoisomerase II inhibitors, and irradiation. J Clin Oncol 11:1039–1045
Schiavetti A, Varrasso G, Maurizi P, Castello MA (2001) Two secondary leukemias among 15 children given oral etoposide. Med Pediatr Oncol 37:148–149
Zhang Y, Rowley JD (2006) Chromatin structural elements and chromosomal translocations in leukemia. DNA Repair (Amst) 5:1282–1297. doi:10.1016/j.dnarep.2006.05.020
Liu LF, Rowe TC, Yang L, Tewey KM, Chen GL (1983) Cleavage of DNA by mammalian DNA topoisomerase II. J Biol Chem 258:15365–15370
Tanaka T, Halicka HD, Traganos F, Seiter K, Darzynkiewicz Z (2007) Induction of ATM activation, histone H2AX phosphorylation and apoptosis by etoposide: relation to cell cycle phase. Cell Cycle 6:371–376
Dartsch DC, Gieseler F (2007) Repair of idarubicin-induced DNA damage: a cause of resistance? DNA Repair (Amst) 6:1618–1628. doi:10.1016/j.dnarep.2007.05.007
Fan JR, Peng AL, Chen HC, Lo SC, Huang TH, Li TK (2008) Cellular processing pathways contribute to the activation of etoposide-induced DNA damage responses. DNA Repair (Amst) 7:452–463. doi:10.1016/j.dnarep.2007.12.002
Agner J, Falck J, Lukas J, Bartek J (2005) Differential impact of diverse anticancer chemotherapeutics on the Cdc25A-degradation checkpoint pathway. Exp Cell Res 302:162–169. doi:10.1016/j.yexcr.2004.08.035
Hartlerode AJ, Scully R (2009) Mechanisms of double-strand break repair in somatic mammalian cells. Biochem J 423:157–168. doi:10.1042/BJ20090942
Pardo B, Gomez-Gonzalez B, Aguilera A (2009) DNA repair in mammalian cells: DNA double-strand break repair: how to fix a broken relationship. Cell Mol Life Sci 66:1039–1056. doi:10.1007/s00018-009-8740-3
Shrivastav M, De Haro LP, Nickoloff JA (2008) Regulation of DNA double-strand break repair pathway choice. Cell Res 18:134–147. doi:10.1038/cr.2007.111
Sonoda E, Hochegger H, Saberi A, Taniguchi Y, Takeda S (2006) Differential usage of non-homologous end-joining and homologous recombination in double strand break repair. DNA Repair (Amst) 5:1021–1029. doi:10.1016/j.dnarep.2006.05.022
Flygare J, Benson F, Hellgren D (1996) Expression of the human RAD51 gene during the cell cycle in primary human peripheral blood lymphocytes. Biochim Biophys Acta 1312:231–236
Mao Z, Bozzella M, Seluanov A, Gorbunova V (2008) DNA repair by nonhomologous end joining and homologous recombination during cell cycle in human cells. Cell Cycle 7:2902–2906
Daley JM, Wilson TE (2005) Rejoining of DNA double-strand breaks as a function of overhang length. Mol Cell Biol 25:896–906. doi:10.1128/MCB.25.3.896-906.2005
Hansen L, Lundin C, Spang-Thomsen M, Petersen L, Helleday T (2003) The role of Rad51 in etoposide (VP-16) resistance in small cell lung cancer. Int J Cancer 105:472–479. doi:10.1002/ijc.11106
Iliakis G, Wang H, Perrault AR, Boecker W, Rosidi B, Windhofer F, Wu W, Guan J, Terzoudi G, Pantelias G (2004) Mechanisms of DNA double strand break repair and chromosome aberration formation. Cytogenet Genome Res 104:14–20. doi:10.1159/000077461
Wang H, Zeng Z-C, Bui T, Sonoda E, Takata M, Takeda S, Iliakis G (2001) Efficient rejoining of radiation-induced DNA double-strand breaks in vertebrate cells deficient in genes of the RAD52 epistasis group. Oncogene 20:2212–2224
Fiorenza MT, Bevilacqua A, Bevilacqua S, Mangia F (2001) Growing dictyate oocytes, but not early preimplantation embryos, of the mouse display high levels of DNA homologous recombination by single-strand annealing and lack DNA nonhomologous end joining. Dev Biol 233:214–224. doi:10.1006/dbio.2001.0199
Goedecke W, Eijpe M, Offenberg HH, van Aalderen M, Heyting C (1999) Mre11 and Ku70 interact in somatic cells, but are differentially expressed in early meiosis. Nat Genet 23:194–198. doi:10.1038/13821
Hamer G, Roepers-Gajadien HL, van Duyn-Goedhart A, Gademan IS, Kal HB, van Buul PP, Ashley T, de Rooij DG (2003) Function of DNA-protein kinase catalytic subunit during the early meiotic prophase without Ku70 and Ku86. Biol Reprod 68:717–721. doi:10.1095/biolreprod.102.008920
Hopfner K, Putnam C, Tainer J (2002) DNA double-strand break repair from head to tail. Curr Opin Struct Biol 12:115–122. doi:10.1016/S0959-440X(02)00297-X
Khanna K, Jackson S (2001) DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet 27:247–254. doi:10.1038/85798
Van Dyck E, Stasiak A, Stasiak A, West S (1999) Binding of double strand breaks in DNA by human Rad52 protein. Nature 398:728–731. doi:10.1038/19560
Heyer W-D, Li X, Rolfsmeier M, Zhang X-P (2006) Rad54: the Swiss army knife of homologous recombination? Nucl Acid Res 34:4115–4125. doi:10.1093/nar/gkl481
Morin PJ, Vogelstein B, Kinzler KW (1996) Apoptosis and APC in colorectal tumorigenesis. Proc Natl Acad Sci USA 93:7950–7954
Aydiner A, Koyuncu H, Tas F, Topuz E, Disci R (2000) Crossover clinical study comparing the pharmacokinetics of etoposide (75 mg) administered as 25-mg capsules three times a day versus once a day. Int J Clin Pharm Res 20:21–30
Eksborg S, Strandler H-S, Edsmyr F, Näslund I, Tahvanainen P (1985) Pharmacokinetic study of IV infusions of adriamycin. Eur J Clin Pharmacol 28:205–212
Greene R, Collins J, Jenkins J, Speyer J, Myers C (1983) Plasma pharmacokinetics of adriamycin and adriamycinol: implications for the design of in vitro experiments and treatment protocols. Cancer Res 43:3417–3421
Holthuis J, Postmus P, Van Oort W, Hulshoff B, Verleun H, Sleijfer D, Mulder N (1986) Pharmacokinetics of high dose etoposide (VP 16–213). Eur J Cancer Clin Oncol 22:1149–1155
Mross K, Mayer U, Hamm K, Burk K, Hossfeld D (1990) Pharmacokinetics and metabolism of iodo-doxorubicin and doxorubicin in humans. Eur J Clin Pharmacol 39:507–513
van der Gaast A, Vlastuin M, Kok T, Splinter T (1992) What is the optimal dose and duration of treatment with etoposide ? II. Comparative pharmacokinetic study of three schedules: 1 × 100 mg, 2 × 50 mg, and 4 × 25 mg of oral etoposide daily for 21 days. Semin Oncol 19:8–12
Zhu G, Gilchrist R, Borley N, Chng H, Morgan M, Marshall J, Camplejohn R, Muir G, Hart I (2004) Reduction of TSG101 protein has a negative impact on tumor cell growth. Int J Cancer 109:541–547. doi:10.1002/ijc.20014
Dartsch D, Schaefer A, Boldt S, Kolch W, Marquardt H (2002) Comparison of anthracycline-induced death of human leukemia cells: programmed cell death versus necrosis. Apoptosis 7:537–548. doi:10.1023/A:1020647211557
Singh N, McCoy M, Tice R, Schneider E (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191. doi:10.1016/0014-4827(88)90265-0
Collins AR, Oscoz AA, Brunborg G, Gaivao I, Giovannelli L, Kruszewski M, Smith CC, Stetina R (2008) The comet assay: topical issues. Mutagenesis 23:143–151. doi:10.1093/mutage/gem051
Baldwin EL, Osheroff N (2005) Etoposide, topoisomerase II and cancer. Curr Med Chem Anticancer Agents 5:363–372
Chen G, Yang L, Rowe T, Halligan B, Tewey K, Liu L (1984) Nonintercalative antitumor drugs interfere with the breakage reunion reaction of mammalian DNA topoisomerase II. J Biol Chem 259(21):13560–13566
Hande K (1998) Etoposide: four decades of development of a topoisomerase II inhibitor. Eur J Cancer 34:1514–1521. doi:10.1016/S0959-8049(98)00228-7
Brown JM, Wilson G (2003) Apoptosis genes and resistance to cancer therapy: what does the experimental and clinical data tell us? Cancer Biol Ther 2:477–490
Brown JM, Wouters BG (1999) Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res 59:1391–1399
Lo Nigro C, Arnolfo E, Taricco E, Fruttero A, Russi EG, Lucio F, Ribero S, Comino A, Merlano M, Ungari S (2007) The cisplatin-irradiation combination suggests that apoptosis is not a major determinant of clonogenic death. Anticancer Drugs 18:659–667. doi:10.1097/CAD.0b013e328087388f
Russell J, Ling CC (2003) Studies with cytotoxic agents suggest that apoptosis is not a major determinant of clonogenic death in neuroblastoma cells. Eur J Cancer 39:2234–2238. doi:10.1016/S0959-8049(03)00488-X
Bertrand R, Sarang M, Jenkin J, Kerrigan D, Pommier Y (1991) Differential induction of secondary DNA fragmentation by topoisomerase II inhibitors in human tumor cell lines with amplified c-myc expression. Cancer Res 51:6280–6285
Gieseler F, Nussler V, Brieden T, Kunze J, Valsamas S (1998) Intracellular pharmacokinetics of anthracyclines in human leukemia cells: correlation of DNA-binding with apoptotic cell death. Int J Clin Pharmacol Ther 36:25–28
Li TK, Liu LF (2001) Tumor cell death induced by topoisomerase-targeting drugs. Annu Rev Pharmacol Toxicol 41:53–77. doi:10.1146/annurev.pharmtox.41.1.53
Long BH, Musial ST, Brattain MG (1985) Single- and double-strand DNA breakage and repair in human lung adenocarcinoma cells exposed to etoposide and teniposide. Cancer Res 45:3106–3112
Chatterjee S, Trivedi D, Petzold S, Berger N (1990) Mechanism of epipodophyllotoxin-induced cell death in poly(adenosine diphosphate-ribose) synthesis-deficient V79 Chinese hamster cell lines. Cancer Res 50:2713–2718
Sung PA, Libura J, Richardson C (2006) Etoposide and illegitimate DNA double-strand break repair in the generation of MLL translocations: new insights and new questions. DNA Repair (Amst) 5:1109–1118. doi:10.1016/j.dnarep.2006.05.018
Chuang SM, Wang LH, Hong JH, Lin YW (2008) Induction of Rad51 protein levels by p38 MAPK decreases cytotoxicity and mutagenicity in benzo[a]pyrene-exposed human lung cancer cells. Toxicol Appl Pharmacol 230:290–297. doi:10.1016/j.taap.2008.03.001
Averbeck D, Averbeck S (1998) DNA photodamage, repair, gene induction and genotoxicity following exposures to 254 nm UV and 8-methoxypsoralen plus UVA in a eukaryotic cell system. Photochem Photobiol 68:289–295. doi:10.1111/j.1751-1097.1998.tb09683.x
Cohen Y, Dardalhon M, Averbeck D (2002) Homologous recombination is essential for RAD51 up-regulation in Saccharomyces cerevisiae following DNA crosslinking damage. Nucleic Acids Res 30:1224–1232
Klein HL (2008) The consequences of Rad51 overexpression for normal and tumor cells. DNA Repair (Amst) 7:686–693. doi:10.1016/j.dnarep.2007.12.008
Karran P (2000) DNA double strand break repair in mammalian cells. Curr Opin Genet Dev 10:144–150. doi:10.1016/S0959-437X(00)00069-1
Han Z, Malik N, Carter T, Reeves WH, Wyche JH, Hendrickson EA (1996) DNA-dependent protein kinase is a target for a CPP32-like apoptotic protease. J Biol Chem 271:25035–25040. doi:10.1074/jbc.271.40.25035
Kim S, Kim D, Han J, Jeong C, Chung B, Kang C, Li G (1999) Ku autoantigen affects the susceptibility to anticancer drugs. Cancer Res 59:4012–4017
Smith G, Jackson S (1999) The DNA-dependent protein kinase. Genes Dev 13:916–934
Song Q, Lees-Miller SP, Kumar S, Zhang Z, Chan DW, Smith GC, Jackson SP, Alnemri ES, Litwack G, Khanna KK, Lavin MF (1996) DNA-dependent protein kinase catalytic subunit: a target for an ICE-like protease in apoptosis. EMBO J 15:3238–3246
Zhu P, Zhang D, Chowdhury D, Martinvalet D, Keefe D, Shi L, Lieberman J (2006) Granzyme A, which causes single-stranded DNA damage, targets the double-strand break repair protein Ku70. EMBO Rep 7:431–437. doi:10.1038/sj.embor.7400622
Gama V, Yoshida T, Gomez JA, Basile DP, Mayo LD, Haas AL, Matsuyama S (2006) Involvement of the ubiquitin pathway in decreasing Ku70 levels in response to drug-induced apoptosis. Exp Cell Res 312:488–499. doi:10.1016/j.yexcr.2005.11.016
Lee SE, Moore JK, Holmes A, Umezu K, Kolodner RD, Haber JE (1998) Saccharomyces Ku70, mre11/rad50 and RPA proteins regulate adaptation to G2/M arrest after DNA damage. Cell 94:399–409. doi:10.1016/S0092-8674(00)81482-8
Zhang Y, Hefferin ML, Chen L, Shim EY, Tseng HM, Kwon Y, Sung P, Lee SE, Tomkinson AE (2007) Role of Dnl4-Lif1 in nonhomologous end-joining repair complex assembly and suppression of homologous recombination. Nat Struct Mol Biol 14:639–646. doi:10.1038/nsmb1261
Frank-Vaillant M, Marcand S (2002) Transient stability of DNA ends allows nonhomologous end joining to precede homologous recombination. Mol Cell 10:1189–1199. doi:10.1016/S1097-2765(02)00705-0
Ira G, Pellicioli A, Balijja A, Wang X, Fiorani S, Carotenuto W, Liberi G, Bressan D, Wan L, Hollingsworth NM, Haber JE, Foiani M (2004) DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature 431:1011–1017. doi:10.1038/nature02964
Acknowledgments
We would like to thank Prof. Dr. Frank Gieseler from the University Hospital Luebeck for many helpful discussions. Our work was supported by the Ernst und Elfriede Griebel’s Foerderungs- und Unterstuetzungsstiftung, Hamburg. This paper is written in memory of Michael R. Clark.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schonn, I., Hennesen, J. & Dartsch, D.C. Cellular responses to etoposide: cell death despite cell cycle arrest and repair of DNA damage. Apoptosis 15, 162–172 (2010). https://doi.org/10.1007/s10495-009-0440-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-009-0440-9