Abstract
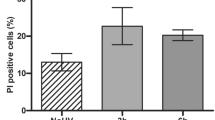
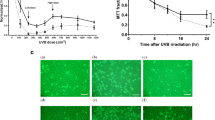
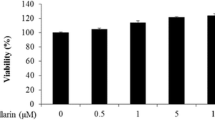
The induction of apoptosis in keratinocytes by ultraviolet (UV)-irradiation is considered to be a protective function against skin cancer. UV-induced DNA damage is a crucial event in UVB- and UVC-mediated apoptosis. However, the differences between the UVB- and UVC-induced apoptotic pathways remain unclear. Here we examine the differential mechanisms by which UVB and UVC irradiations induce keratinocyte apoptosis using human keratinocyte HaCaT cells. Differences in the production of (6-4)photoproducts ((6-4)PPs) and cyclobutane pyrimidine dimers (CPDs) were measured following irradiation with UVB and UVC at doses causing the same extent of apoptotic cell death. In addition, main apoptotic features, such as caspase activation and its regulation, were compared between UVB- and UVC-induced apoptosis. Exposures of 500 J/m2 UVB and 100 J/m2 UVC resulted in apoptosis to almost the same extent. At these apoptotic doses, the amounts of both (6-4)PPs and CPDs were significantly larger in the case of UVC irradiation than UVB irradiation; in parallel, the release of cytochrome c and Smac/DIABLO and the activation of caspases-9 following UVC irradiation were greater than after UVB irradiation. Importantly, caspase-8 activation occurred only in UVB-irradiated cells. Furthermore, the activation of caspase-8 was not inhibited by caspases-9 and -3 specific tetrapeptide inhibitors, indicating that the caspase-8 cleavage is not due to feedback from activation of caspases-9 and -3. Thus, these results clearly suggest that the reason apoptosis is induced to the same extent by UVB irradiation as by UVC irradiation, despite the lower production of photoproducts in DNA by UVB irradiation, is attributable to the additional activation of the caspase-8 pathway. Thus, UVB irradiation induces apoptosis through both mitochondrial (intrinsic) and caspase-8 activation (extrinsic) pathways, while UVC induces apoptosis only via the intrinsic pathway.
Similar content being viewed by others
References
Ananthaswamy HN, Pierceall WE. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol 1990; 52: 1119–1136.
Beissert S, Schwarz T. Mechanisms involved in ultraviolet light-induced immunosuppressionit. J Invest Dermatol Symp Proc 1999; 4: 61–64.
Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Mechanisms of photoaging and chronological skin aging. Arch Dermatol 2002; 138: 1462–1470.
de Gruijl FR, van Kranen HJ, Mullenders LH. UV-induced DNA damage, repair, mutations and oncogenic pathways in skin cancer. J Photochem Photobiol B 2001; 63: 19–27.
Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, Tsuru K, Horikawa T. UV-induced skin damage. Toxicology 2003; 189: 21–39.
Cohen JJ. Apoptosis. Immunol Today 1993; 14: 126–130.
Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972; 26: 239–257.
Wyllie AH, Kerr JF, Currie AR. Cell death: The significance of apoptosis. Int Rev Cytol 1980; 68: 251–306.
Nunez G, Benedict MA, Hu Y, Inohara N. Caspases: The proteases of the apoptotic pathway. Oncogene 1998; 17: 3237–3245.
Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 1999; 15: 269–290.
Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 1994; 371: 346–347.
Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 1998; 391: 43–50.
Shiokawa D, Ohyama H, Yamada T, Tanuma S. Purification and properties of DNase gamma from apoptotic rat thymocytes. Biochem J 1997; 326: 675–681.
Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 2001; 412: 95–99.
Rosenstein BS, Mitchell DL. Action spectra for the induction of pyrimidine(6-4)pyrimidone photoproducts and cyclobutane pyrimidine dimers in normal human skin fibroblasts. Photochem Photobiol 1987; 45: 775–780.
Pfeifer GP. Formation and processing of UV photoproducts: Effects of DNA sequence and chromatin environment. Photochem Photobiol 1997; 65: 270–283.
Aragane Y, Kulms D, Metze D, Wilkes G, Poppelmann B, Luger TA, Schwarz T. Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L. J Cell Biol 1998; 140: 171–182.
Sheikh MS, Antinore MJ, Huang Y, Fornace AJ Jr. Ultraviolet-irradiation-induced apoptosis is mediated via ligand independent activation of tumor necrosis factor receptor 1. Oncogene 1998; 17: 2555–2563.
Sitailo LA, Tibudan SS, Denning MF. Activation of caspase-9 is required for UV-induced apoptosis of human keratinocytes. J Biol Chem 2002; 277: 19346–19352.
Takasawa R, Tanuma S. Sustained release of Smac/DIABLO from mitochondria commits to undergo UVB-induced apoptosis. Apoptosis 2003; 8: 291–299.
Mori T, Nakane M, Hattori T, Matsunaga T, Ihara M, Nikaido O. Simultaneous establishment of monoclonal antibodies specific for either cyclobutane pyrimidine dimer or (6-4)photoproduct from the same mouse immunized with ultraviolet-irradiated DNA. Photochem Photobiol 1991; 54: 225–232.
Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 1988; 106: 761–771.
Nomura K, Imai H, Koumura T, Arai M, Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase suppresses apoptosis mediated by a mitochondrial death pathway. J Biol Chem 1999; 274: 29294–29302.
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997; 91: 479–489.
Cain K, Bratton SB, Langlais C, Walker G, Brown DG, Sun XM, Cohen GM. Apaf-1 oligomerizes into biologically active approximately 700-kDa and inactive approximately 1.4-MDa apoptosome complexes. J Biol Chem 2000; 275: 6067–6070.
Adrain C, Martin SJ. The mitochondrial apoptosome: A killer unleashed by the cytochrome seas. Trends Biochem Sci 2001; 26: 390–397.
Chai J, Du C, Wu JW, Kyin S, Wang X, Shi Y. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature 2000; 406: 855–862.
Srinivasula SM, Datta P, Fan XJ, Fernandes-Alnemri T, Huang Z, Alnemri ES. Molecular determinants of the caspase-promoting activity of Smac/DIABLO and its role in the death receptor pathway. J Biol Chem 2000; 275: 36152– 36157.
Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000; 102: 33–42.
Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 2000; 102: 43–53.
Wood RD. DNA repair in eukaryotes. Annu Rev Biochem 1996; 65: 135–167.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takasawa, R., Nakamura, H., Mori, T. et al. Differential apoptotic pathways in human keratinocyte HaCaT cells exposed to UVB and UVC. Apoptosis 10, 1121–1130 (2005). https://doi.org/10.1007/s10495-005-0901-8
Issue Date:
DOI: https://doi.org/10.1007/s10495-005-0901-8