Abstract
Background: The immunogenicity of the polyvalent tumor cell vaccine CancerVax has been correlated with the survival of patients receiving active immunotherapy for melanoma. Because the various antigens expressed on the vaccine are common to colon adenocarcinoma cells, we examined the survival impact of immune responses elicited by CancerVax in patients with advanced colon cancer refractory to standard therapy.
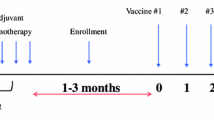
Methods: Twenty-seven patients with American Joint Committee on Cancer (AJCC) stage IV colorectal adenocarcinoma were entered prospectively into the study. CancerVax was coadministered with bacille Calmette-Guerin (BCG) for the first 2 weeks of vaccine treatment. Blood was drawn at the start of therapy and every 2 weeks thereafter to measure serum titers of immunoglobulin (Ig)G and IgM against TA90 (a 90-kD immunogen common to colon cancer and CancerVax cells) and against purified protein derivative (PPD), a nontumor control antigen. Cellular immune responses were evaluated by delayed-type hypersensitivity (DTH) reaction to vaccine cells and to PPD. Mean follow-up time was 17.5 months.
Results: There was a significant (P = .0001) increase in anti-TA90 IgG and IgM titers and in DTH response to vaccine cells. Humoral and skin responses to TA90 did not correlate with responses to PPD (P = .199 for IgM, P = .958 for IgG, and P = .149 for DTH). This suggests that these responses are not a manifestation of general immune competence. The median overall survival (OS) was 21.9 months for the entire group. Overall survival was higher among patients whose IgMTA90 titer was >800 (P = .003) or whose disease-free interval exceeded 12 months (P = .031). Multivariate Cox regression analysis—using age, sex, disease-free interval, disease status, extent of metastasis, humoral responses, and DTH responses—found only peak IgMTA90 titer to be a significant predictor of overall survival (P = .0365).
Conclusions: CancerVax can induce measurable humoral and cellular immune responses to tumor-associated antigens in patients with advanced-stage colon cancer. These responses correlate with overall survival. This novel therapeutic regimen for patients with advanced colon cancer merits further investigation.
Similar content being viewed by others
REFERENCES
Hoon DSB, Irie RF. Current status of human melanoma vaccines: Can they control malignant melanoma? BioDrugs 1997; 7: 66–84.
Morton DL, Foshag LJ, Hoon DSB, et al. Prolongation of survival in metastatic melanoma after active specific immunotherapy with a new polyvalent melanoma vaccine. Ann Surg 1992; 216: 463–82.
Morton DL, Hoon DSB, Nizze JA, et al. Polyvalent melanoma vaccine improves survival of patients with metastatic melanoma. Ann N Y Acad Sci 1993; 690: 120–34.
Takahashi T, Johnson TD, Nishinaka Y, Morton DL, Irie RF. IgM anti-ganglioside antibodies induced by melanoma cell vaccine correlate with survival of melanoma patients. J Invest Dermatol 1999; 112: 101–5.
Hoon DSB, Morisaki T, Uchiyama A, et al. Augmentation of T-cell response with a melanoma cell vaccine expressing specific HLA-A antigens. Ann N Y Acad Sci 1993; 690: 343–5.
Hsueh EC, Famatiga E, Gupta RK, QiK, Morton DL. Enhancement of complement-dependent cytotoxicity by polyvalent melanoma cell vaccine (CancerVax): correlation with survival. Ann Surg Oncol 1998; 5: 595–602.
Barth A, Hoon DSB, Foshag LJ, et al. Polyvalent melanoma cell vaccine induces delayed-type hypersensitivity and in-vitro cellular immune response. Cancer Res 1994; 54: 3342–5.
Euhus DM, Gupta RK, Morton DL. Isolation and characterization of 90–100 kDa tumor-associated antigen in the sera of melanoma patients. Int J Cancer 1990; 45: 1065–70.
Jones RC, Kelley M, Gupta RK, et al. Immune response to polyvalent melanoma cell vaccine in AJCC stage III melanoma: an immunologic survival model. Ann Surg Oncol 1996; 3: 437–45.
Hsueh EC, Gupta RK, QiK, Yee R, Leopoldo Z, Morton DL. TA90 immune complex predicts survival following surgery and adjuvant vaccine immunotherapy for stage IV melanoma. Cancer J Sci Am 1997; 3: 364–70.
Hsueh EC, Gupta RK, Morton DL. Correlation of specific immune responses with survival in melanoma patients with distant metastases receiving polyvalent melanoma cell vaccine. J Clin Oncol 1998; 16: 2913–20.
Gupta RK, Morton DL. Monoclonal antibody-based ELISA to detect glycoprotein tumor-associated-antigen-specific immune complexes in cancer patients. J Clin Lab Anal 1992; 6: 329–36.
Habal N, Gupta RK, Bilchik AJ, Che A, Morton DL. TA90-IC, a new marker for colon cancer. Presented at: 52nd Annual Cancer Symposium of the Society for Surgical Oncology; March 4–7, 1999; Orlando, Florida.
Morton DL, Foshag LJ, Nizze JA, et al. Active specific immunotherapy in malignant melanoma. Semin Surg Oncol 1989; 5: 420–25.
Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–14.
Fong Y, Blumgart LH. Hepatic colorectal metastasis: current status of surgical therapy. Oncology 1998; 12: 1489–98.
Geoghegan JG, Scheele J. Treatment of colorectal liver metastases. Br J Surg 1999; 86: 158–69.
Benson AB. Therapy for advanced colorectal cancer. Semin Oncol 1998; 25(5 Suppl 11): 2–11.
Punt CJ. New drugs in the treatment of colorectal carcinoma. Cancer 1998; 83: 679–89.
McCarty TM, Kuhn JA. Cryotherapy for liver tumors. Oncology 1998; 12: 979–87.
Lenciono R, Goletti O, Armillota N, et al. Radio-frequency thermal ablation of liver metastases with a cooled-tip electrode needle: results of a pilot clinical trial. Eur Radiol 1998; 8: 1205–11.
Tellez C, Benson AB, Lyster M, et al. Phase II trial of chemoembolization for the treatment of metastatic colorectal carcinoma to the liver and review of the literature. Cancer 1998; 82: 1250–59.
Holcombe RF, LiA, Stewart RM. Levamisole and interleukin-2 for advanced malignancy. Biotherapy 1998; 11: 255–8.
Morton BA, O’Connor-Tressel M, Beatty BG, Shively JE, Beatty JD. Artifactual CEA elevation due to human anti-mouse antibodies. Arch Surg 1988; 123: 1242–6.
Sakahara H, Saga T, Onodera H, et al. Anti-murine antibody response to mouse monoclonal antibodies in cancer patients. Jpn J Cancer Res 1997; 88: 895–9.
Meredith RF, Khazaeli MB, Plott WE, et al. Phase II study of dual-isotope 131I-labeled monoclonal antibody therapy with interferon in patients with metastatic colorectal cancer. Clin Cancer Res 1996; 2: 1811–8.
Wong JY, Chu DZ, Yamauchi D, et al. Dose escalation trial of indium-111-labeled anti-carcinoembryonic antigen chimeric monoclonal antibody (chimeric T84.66) in presurgical colorectal cancer patients. J Nucl Med 1998; 39: 2097–3104.
Thompson JA, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal 1991; 5: 344–66.
Conry RM, LoBuglio AF, Curiel DT. Polynucleotide-mediated immunization of cancer. Semin Oncol 1996; 23(1): 135–47.
Foon KA, Chakraborty M, John WJ, Sherratt A, Kohler H, Bhattacharya-Chatterjee M. Immune response to the carcinoembryonic antigen in patients treated with an anti-idiotype antibody vaccine. J Clin Invest 1995; 96: 334–42.
Zbar AP, Lemoine NR, Wadhwa M, Thomas H, Snary D, Kmiot WA. Biological therapy: approaches in colorectal cancer. Strategies to enhance carcinoembryonic antigen (CEA) as an immunogenic target. Br J Cancer 1998; 77: 683–93.
Foon KA, John WJ, Chakraborty M, et al. Clinical and immune responses in advanced colorectal cancer patients treated with anti-idiotype monoclonal antibody vaccine that mimics the carcinoembryonic antigen. Clin Cancer Res 1997; 3: 1267–76.
Samanci A, YiQ, Fagerberg J, et al. Pharmacological administration of granulocyte/macrophage colony-stimulating factor is of significant importance for the induction of a strong humoral and cellular response in patients immunized with recombinant carcinoembryonic antigen. Cancer Immunol Immunother 1998; 47: 131–42.
Foon KA, John WJ, Chakraborty M, et al. Clinical and immune responses in resected colon cancer patients treated with anti-idiotype monoclonal antibody vaccine that mimics the carcinoembryonic antigen. J Clin Oncol 1999; 17: 2889–95.
Ockert D, Schirrmacher V, Beck N, et al. Newcastle disease virus-infected intact autologous tumor cell vaccine for adjuvant active specific immunotherapy of resected colorectal carcinoma. Clin Cancer Res 1996; 2: 21–28.
Vermorken JB, Claessen AM, van Tinteren H, et al. Active specific immunotherapy for stage II and stage III human colon cancer: a randomized trial. Lancet 1999; 353: 345–50.
Harris JE, Ryan L, Hoover HC, et al. Active specific immunotherapy for stage II and stage III human colon cancer with an autologous tumor cell vaccine: Eastern Cooperative Oncology Group study E5283. J Clin Oncol 2000; 18: 148–57.
DiFronzo LA, Gupta R, Essner R, et al. An enhanced humoral immune response predicts improved disease-free and overall survival in AJCC stage II melanoma patients receiving polyvalent melanoma vaccine (CancerVax). Proc Am Soc Clin Oncol 1999; 18: 432a
Gough I. Serum immunoglobulins in colorectal cancer. Aust N Z J Surg 1981; 51: 440–42.
Jones SL, Pihl E, Cuthbertson AM, Hughes ES, Johnson WR, Rollo AJ. Immunoglobulins intrinsic to colorectal carcinoma: an unfavorable prognostic association with IgM. J Natl Cancer Inst 1983; 71: 469–71.
Slater G, Papatestas AE, Shafir M, Aufses AH. Serum immunoglobulins in colorectal cancer. J Surg Oncol 1980; 14: 167–71.
Agrez MV. Cell adhesion molecules and colon cancer. Aust N Z J Surg 1996; 66: 791–98.
Huston DP. The biology of the immune system. JAMA 1997; 278: 1804–14.
Hunt KK, Shibata M, Gupta RK, Morton DL. Complement-dependent lysis of tumor cells by a baboon IgM antibody to a tumor-associated antigen. Cancer Immunol Immunother 1992; 34: 377–82.
Takahashi T, Johnson TD, Nishinaka Y, Morton DL, Irie RF. IgM anti-ganglioside antibodies induced by melanoma cell vaccine correlate with survival of melanoma patients. J Invest Dermatol 1999; 112: 205–9.
Jones PC, Sze LL, Liu PY, Morton DL, Irie RF. Prolonged survival for melanoma patients with elevated IgM antibody to oncofetal antigen. J Natl Cancer Inst 1981; 66: 249–54.
Livingston PO. Approaches to augmenting the immunogenicity of melanoma gangliosides: from whole melanoma cells to ganglioside-KLH conjugate vaccine. Immunol Rev 1995; 145: 147–66.
Chang A. Colorectal cancer.In: Greenfield LJ, Mulholland MW, Oldham KT, Zelenock GB, eds. Surgery Scientific Principles and Practice. Philadelphia: JB Lippincott Company, 1993: 1023.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure: Donald L. Morton has an ownership interest in patents on the vaccine and in the company which has the rights to the vaccine. (Per copyright form date 7/25/00).
Rights and permissions
About this article
Cite this article
Habal, N., Gupta, R.K., Bilchik, A.J. et al. CancerVax, An Allogeneic Tumor Cell Vaccine, Induces Specific Humoral and Cellular Immune Responses in Advanced Colon Cancer. Ann Surg Oncol 8, 389–401 (2001). https://doi.org/10.1007/s10434-001-0389-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10434-001-0389-6