Abstract
The aim of this study was to evaluate the effectiveness of a fibrinogen and thrombin-coated hemostatic sponge (TachoSil®) in the prevention of postsurgical adhesions in a laparoscopic rat model and its histological effects on uterine and peritoneal parenchyma. Design was a prospective randomized blinded study. Setting was in International Laparoscopic Surgery Centre, Clermont-Ferrand, France. There were 100 sexually mature female Sprague–Dawley rats weighing 240 to 300 g and aged 6–8 weeks. A standardized severe surgical injury induced by scissors and 40-W bipolar coagulation in the rat uterine horn, corpus, and peritoneum was performed to induce adhesion formation. After trauma, group 1 (n = 50) received no treatment and group 2 rats (n = 50) received TachoSil® applied on injured areas. Twelve weeks after the procedure, repeat laparoscopy was performed and adhesions were scored according to their extent and severity. A hysterectomy and a peritoneal biopsy in the injured area were achieved by laparotomy in order to investigate on a possible earlier effect of TachoSil® on the uterine and peritoneal parenchyma in 49 rats of each group. TachoSil® group adhesion scores showed a significant decrease on the three injured areas: peritoneum (12.96 vs. 21.66), uterine horn (7.22 vs. 15.20), and uterine corpus (5.88 vs. 34.52). TachoSil® group also demonstrated a major decline of uterine fibrosis and inflammation. This study revealed that TachoSil®, an absorbable biomaterial, can reduce postoperative adhesions after laparoscopic surgery on a rat model. TachoSil® also prevents thermo-induced injuries on uterine parenchyma (less fibrosis and less inflammation).
Similar content being viewed by others
Introduction
Postoperative surgical adhesions in abdominal and pelvic surgery can have a major impact on patient’s subsequent health [1]. These continue to be a significant cause of female infertility, bowel obstruction, chronic pelvic pain, and difficulties at the time of reoperation [2]. Almost 90% of abdominal adhesions seem to be due to surgical antecedents. Despite the development of many surgical techniques and the use of several products to prevent or minimize the formation of adhesions following surgery, at least, 50% of patients still develop substantial adhesions [3]. The aim of our study was to evaluate the ability of a hemostatic sponge on the reduction of peritoneal and uterine adhesions in a laparoscopic rat model. TachoSil®, a particularly promising hemostatic agent, is a bioabsorbable fibrinogen and thrombin-coated hemostatic sponge manufactured by Nycomed Laboratory (Copenhagen, Denmark). We hypothesized that under conditions of inadequate hemostasis, it may contribute to decrease adhesion development in a laparoscopic rat model. TachoSil® may also prevent thermo-induced injuries on peritoneum and uterine parenchyma.
Materials and methods
The bioabsorbable TachoSil® sponge is an equine collagen sponge coated with human fibrinogen and human thrombin (5.5 mg and 2.0 IU per cm2, respectively). These two components of the coating dissolve and partly diffuse into the wound surface at contact with physiological fluids like blood or lymph. A fibrinogen–thrombin reaction follows mimicking the last phase of blood coagulation leading to the conversion of fibrinogen into fibrin monomers. This spontaneously polymerize to a fibrin stable clot holding the collagen tightly to the wound surface, preventing bleeding and sealing the tissue.
The experimental design was a randomized, blinded, controlled, parallel-group study. The animals were treated in accordance with recommendations from the National Institutes of Health Guidelines for the Use of Animals in Research and were approved by the University Animal Care and Use Committee. Sexually mature female Sprague–Dawley rats, aged 6–8 weeks and weighing 240–300 g, were housed for a minimum of 1 week before surgery. The animals were maintained under standard laboratory conditions (temperature 20–22°C, relative humidity 50–70%, 12:12 h light/dark ratio) and were fed a standard laboratory diet and had free access to food and water before and after the operative procedures. In the event of a rat dying before the second operative procedure, the rat was replaced for laparoscopy in order to maintain the same number of rats in each group. Before each procedure, all instruments were sterilized by rubbing with 70% isopropanol. On the day of the surgery, each rat was anesthetized with a single intraperitoneal injection of pentobarbital at 0.08 mg/g (Nembutal, Sanofi Santé Animale, Brussels, Belgium) and was branded (metallic clip on ear and color marking on tail). Rats were placed in dorsal recumbent position, abdominal operative surface was shaved (electric shaver), and disinfection of the abdominal operative surface was carried out with dermic polyvidone (Viatris, Mérignac, France).
A 5-mm abdominal incision under the xiphoid process permitting the introduction of a 14-G Veress needle (Tyco Healthcare, Norwalk, CT, USA) was performed. After safety tests, abdominal pneumoperitoneum was obtained with CO2 gas at a pressure of 5 mmHg and a flow rate of 0.5 L/min with an automated insufflator (Electronic CO2-Endoflator®, Karl Storz, Tuttlingen, Germany) using pure CO2 gas at room temperature. As soon as insufflation was achieved, the needle was removed, and a 5.5-mm transparent trocar with insufflation port (Vectec, Vichy, France) was introduced through the same incision, permitting the insertion of a 4-mm 10° endoscope connected to a single-chip video camera (Karl Storz Digicam) and a xenon light source (Karl Storz, Tuttlingen, Germany). Pneumoperitoneum was maintained at 5 mmHg and the rat was immobilized in Trendelenburg position. A second incision of 3 mm was performed in the right hypochondriac region under laparoscopic guidance, allowing the introduction of a 5.5-mm operative trocar with transparent cannula (Vectec, Vichy, France) for the introduction of instruments (laparoscopic scissors, atraumatic forceps, and bipolar coagulation). Another 8-mm incision was realized in the left hypochondriac region in order to introduce a 10-mm operative trocar with reduction device for 5 mm instrument (Vectec, Vichy, France) for the placement of the sponge. All trocars were fixed to the abdominal wall by a 2/0 monofilament nylon nonabsorbable suture (Monosof® 2/0, Tyco Healthcare, USA).
Rats were randomly assigned to two groups: group 1 (control group: without treatment) and group 2 (injured areas covered by TachoSil®).
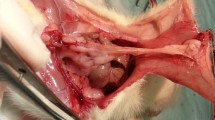
After complete inspection of the abdominopelvic cavity, visualization of the uterine horns required displacement of bowel with the grasping forceps. A standardized severe injury consisting in partial section of the uterine corpus, uterine horn (after randomization of the side), and denuding a 2-cm2 area of the abdominal peritoneum sidewall (centered on a blood vessel) was performed with scissors. Then, an application of 40-W current for 2 s using bipolar forceps (Autocon 350®, Karl Storz) was carried out in order to coagulate the three operative sites in group 1 rats. In group 2 rats, after bipolar coagulation of the peritoneum and the uterine horn, a piece of TachoSil® (1 × 1.5 cm) was put into the injured areas. Thus, in this group, the uterine corpus was not electrocoagulated after section but benefited only from a piece of TachoSil® (Fig. 1).
Because CO2 pneumoperitoneum is a cofactor of adhesion formation, it was maintained for a period of 30 min. In order to create a conventional laparoscopic surgery environment with 100% of carbon dioxide, a 26-G needle (20 mL/min) was inserted under laparoscopic guidance in the right iliac fosse (Becton Dickinson, Sandy, UT, USA). At the end of the procedure, the sutures around trocars were removed and CO2 was allowed to escape spontaneously from the peritoneal cavity. The peritoneum was not sutured. The abdominal wall was nicked using undyed braided absorbable suture (Coated Vicryl® 3/0, Ethicon®, Johnson & Johnson). The rats were placed under a heating lamp for 2 h in order to avoid caloric loss resulting from anesthesia.
A second-look laparoscopy was performed through the same incisions 12 weeks after the initial surgery to evaluate the incidence of postoperative adhesions and their severity. The results were documented on video tape (only surgical sites were evaluated) and assessed by two surgeons unfamiliar with the initial operative site, to prevent evaluator bias. The postoperative adhesions were scored according to their extent and severity using qualitative and quantitative parameters according to modified Mage’s scoring system [4] (Table 1). The recorded films were screened (the two evaluators used the same monitor) in front of a scanned millimeter graph A4 paper (21 × 29.7 cm) in order to create a spatial resolution similar to a pixelization. We compared the size of the adhesion with one of the forceps grasp which represents a fixed data (evaluation of a percentage). In the event of disagreement over surgeon’s scores, the higher score was used in calculations.
Medians and ranges of the adhesion scores were calculated for each group and operative site separately. For the comparison of groups, insofar as each rat has three operative sites (peritoneum, uterine horn, and uterine corpus), we compared them 2 to 2. The median values were obtained including the sites without adhesion. The assigned score when no adhesions were detected was 1 for each operative site.
The final procedure consisted in hysterectomy with bilateral salpingo-oophorectomy and in peritoneal biopsy in the injured area, achieved by a median laparotomy in order to investigate on a possible earlier effect of the fleece-bound sealing system on the uterine and peritoneal parenchyma in 49 rats of each group (after hematoxylin erythrosin saffron coloration; Fig. 3). The evaluated uterine and peritoneal histological criterions were semiquantitative for inflammation, fibrosis, necrosis, vascular congestion, hemosiderin deposit (absence, 0; low, 1–15%; moderate, 16–49%; severe, 50–100%), and qualitative for granuloma (presence or absence). The histological study was performed by an experienced pathologist (C. Darcha). Euthanasia with CO2 inhalation closed the procedure.
Statistical analysis was carried out using the Stat View System® (SAS-Institute, Cary, NC, USA). The comparison of adhesion scores was performed using a nonparametric Kruskal–Wallis test. The histological data were compared using chi-squared test. A statistical significant was considered for p < 0.05.
Results
One hundred nineteen animals were used for the experiment, 100 rats undergoing a total of 298 procedures (200 laparoscopic and 98 laparotomic). Nineteen rats died until postoperative day 9 and were excluded from the analysis: Two died during the anesthetic induction before randomization, one peroperatively in group 2 (hemorrhagic complication during the placement of the first trocar), and 16 in postoperative period (all cases occurred before postoperative day 9). The number of animals dying in each group was respectively seven (14%) and nine (18%). No death occurred over postoperative day 9. In the end, 50 rats in each group were kept for the study.
The weight variation showed a mean increase of 50.3 g in group 1 and 45.5 g in group 2 during the observation period.
TachoSil® group adhesion scores showed a statistically significant decrease—26.06 vs. 71.38 (p < 0.0001). The adhesion scores are presented in Table 2. The results revealed statistical significant lower scores on the three injured areas:
-
Peritoneum—12.96 vs. 21.66 (p = 0.026)
-
Uterine horn—7.22 vs. 15.20 (p = 0.0003)
-
Uterine corpus—5.88 vs. 34.52 (p < 0.0001)
The adhesion scores according to the site are presented in Table 3. We have also noted that the numbers of injured areas presenting vascular adhesions requiring sharp dissection was significantly increased in group 1 (Figs. 2 and 3).
Concerning the histological study, TachoSil® group demonstrates a major decline of uterine fibrosis and inflammation (respectively p = 0.0004 and p = 0.0001). More detailed in this group, we found a statistical significant reduction of uterine granuloma (p = 0.005) and uterine hemosiderin deposit (p = 0.001). On the other hand, there was a significant decrease of peritoneal granuloma in control group (p = 0.013). However, there was no statistical significant difference affecting the other histological criterions between the two groups (peritoneal fibrosis: p = 1, peritoneal inflammation: p = 1, uterine necrosis: p = 1, peritoneal necrosis: p = 0.14, peritoneal hemosiderin deposit: p = 0.64, peritoneal vascular congestion: p = 0.68, and uterine vascular congestion: p = 0.17).
Discussion
The aim of this study was to evaluate the efficacy of TachoSil® to prevent any adhesion formation when severe peritoneal wounds are induced by scissors and bipolar coagulation during laparoscopy in a rat model and to analyze the impact of the fleece-bound system on uterine and peritoneal parenchyma. The choice of laparoscopic procedures was decided regarding to minimally invasive intraperitoneal manipulations and the quality of images obtained by the endoscope (“magnifying effect”) that allows a more accurate evaluation of the adherences.
Bipolar coagulation is commonly used in clinical surgical practice in order to perform hemostasis and ablation of peritoneal lesions. The intensity of the current we used (40 W) in order to cause severe peritoneal injury corresponded to the power frequently used in clinical practice for the hemostasis. Roman et al. obtained adhesions to this level in all cases in a rat laparoscopic model [3]. Adhesiogenesis begins with the development of a fibrin matrix occurring for example during coagulation in suppressed fibrinolysis environment [5, 6]. The use of the TachoSil combipatch in postoperative adhesion prevention could appear possible in this context. Forestier et al. observed that in a rabbit model, peritoneal burn damages decreased with the use of bipolar scissors comparatively to monopolar coagulation [7]. Mecke et al. demonstrated in a rat model that intra-abdominal adhesions were lower than in rats undergoing monopolar or CO2 laser coagulation [8]. Laparoscopy is considered source of less adhesion than laparotomy, in agreement with several studies [9-13]. However, Elkelani et al. [14] confirmed that adhesiogenesis mechanisms were different after laparoscopy and laparotomy. For that purpose, CO2 pneumoperitoneum appears like a cofactor in adhesion formation [9, 15]. Thus, we maintained the pneumoperitoneum for 30 min at the end of each laparoscopic procedure.
Adhesions that develop after abdominopelvic surgery are responsible for significant clinical and economic problems. The use of microsurgical principles and adhesion reduction devices represents the strategic mainstay of adhesion prevention [9, 16]. Several agents have been evaluated in order to reduce them but with inconstant and unsatisfactory results: barrier materials Interceed® (Gynecare-Ethicon, Somerville, NJ, USA) and Seprafilm® (Genzyme, Cambridge, MA, USA), for example, interposed between adjacent surfaces to avoid direct contact, solutions (crystalloids, hyaluronan-based gels, dextran, icodextrin) in attempt to keep adjacent peritoneal surfaces away (hydroflottation), and drugs such as heparin, corticosteroids, anti-inflammatory agents, and statins. It appears clearly that the ideal anti-adhesion agent might present some qualities: It must be biocompatible, biodegradable, noninflammatory, nonimmunogenic, and nonsubject to rapid degradation.
TachoSil® has been used successfully in several indications. It represents a supportive treatment in surgery for the improvement of hemostasis, to promote tissue sealing and for suture support in vascular surgery when standard techniques are insufficient. Anegg et al. [17] reported an application in pulmonary resection: The use of TachoSil® resulted in a reduction in air leakage compared with standard techniques (significant reduction in both the time to chest drain removal and the period of hospitalization). Frena and Martin [18] showed an effective application of the sponge in improvement of intraoperative biliostasis to minimize the risk of postoperative fistula development occurring after hepatic resection. Tagliabue et al. [19] used TachoSil® in patients suffering from severe clotting disturbances in order to perform successful splenectomy and nephrectomy. Some other uses of the coated patch have also been reported: hepatic surgery (liver resection, liver transplantation), urologic surgery (kidney tumor resection, repair of recurrent vesicovaginal fistula), cardiovascular surgery (reduction of postoperative pericardial effusions after aortic tube grafts, in addition to stitches to facilitate mechanical and biologic suture in aortic arch aneurysm repair), digestive surgery (gastrointestinal anastomoses), and neurosurgery (management of cerebrospinal fluid leak after surgical removal of pituitary adenomas) [20-30].
In our study, the animals were reoperated 12 weeks after the initial surgery, corresponding to the period necessary for the hemostatic patch to be completely resorbed, according to the conclusions of Getman et al. who also demonstrated a preventive effect of TachoSil® to prevent pleural adhesions in an experimental rat model [31].
The first aim of our experiment was to determine a possible efficiency of hemostatic fleece to prevent postoperative adhesions in a laparoscopic rat model. TachoSil® group adhesion scores show a statistically significant decrease on the peritoneum, the uterine horn, and the uterine corpus. The fleece-bound sealing system shows an ability to prevent postoperative adhesions in this laparoscopic rat model. More detailed, the adhesion scores were higher for the peritoneum than the uterine corpus and the uterine horn. The uterine corpus was not electrocoagulated but only cut and the effect of TachoSil® appears more beneficial in absence of coagulation, probably due to the preservation of the tissue vascular system.
The second aim of our study was to evaluate a possible earlier effect of TachoSil® on uteroperitoneal parenchyma. Indeed, in clinical practice, the use of bipolar coagulation is very common to perform, for example, a myomectomy. Thus, it is responsible for thermo-induced injuries which can cause postoperative adhesions and parenchymal weakness with subsequent consequences. Agdi and Tulandi advocated a sparing use of electrocautery in surgical management of uterine fibroids to prevent extensive myometrial injuries which can jeopardize myometrial healing and predispose to uterine dehiscence or rupture during pregnancy and labor [32]. In this study, TachoSil® was revealed as a tissue protector insofar as we found a statistically significant decrease of uterine fibrosis and inflammation. However, there was no difference concerning the peritoneal fibrosis and inflammation in groups 1 and 2 because of the use of bipolar coagulation on it (Fig. 4). There was a significant decrease of peritoneal granuloma in control group (p = 0.013) for the same reason. We conclude that the use of TachoSil® in place of bipolar coagulation, especially when the surgical procedure appears particularly hemorrhagic, can offer two advantages: an efficient nondeleterious hemostasis and a potential prevention of adhesion formation by means of shunning the bipolar coagulation. Fedele et al. suggested that bipolar electrocoagulation of the ovarian parenchyma after laparoscopic stripping of an endometriotic ovarian cyst adversely affects ovarian function [33]. Li et al. recently investigated, in a clinical study, the impact of electrocoagulation on ovarian reserve after laparoscopic excision of ovarian cysts. Thus, electrocoagulation is associated with a statistically significant reduction in follicular reserve, which seems partly a consequence of the damage to the ovarian vascular system [34]. TachoSil® might be used in place of it in order to permit a follicular and fertility saving.
Conclusion
These results confirm that TachoSil® has anti-adhesion effects on a laparoscopic rat model. TachoSil® also prevents thermo-induced injuries on uterine parenchyma (less fibrosis and less inflammation). The next step will be to corroborate these data on clinical investigations before extrapolation. Moreover, it seems interesting to study the impact of the fleece-bound sealing system on the ovarian parenchyma in order to show the preventive effect of thermo-induced injuries resulting from electrocoagulation used during ovarian cystectomy.
References
Ellis H, Moran BJ, Thompson JN, Parker MC, Wilson MS, Menzies D, McGuire A, Lower AM, Hawthorn RJ, O’Brien F, Buchan S, Crowe AM (1999) Adhesion related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet 353(9163):1476–1480
Canis M, Botchorishvili R, Wattiez A, Rabischong B, Houlle C, Pouly JL, Manhes H, Bruhat MA (2001) Prevention of peritoneal adhesions. J Gynecol Obstet Biol Reprod (Paris) 30(4):305–324
Roman H, Canis M, Kamble M, Botchorishvili R, Pouly JL, Mage G (2005) Efficacy of three adhesion-preventing agents in reducing severe peritoneal trauma induced by bipolar coagulation in a laparoscopic rat model. Fertil Steril 83(Suppl 1):1113–1118
Mage G, Pouly JL, de Bouquet JJ, Chabrand S, Bruhat MA (1984) Distal tubal obstructions : microsurgery or in vitro fertilization. J Gynecol Obstet Biol Reprod (Paris) 13(8):933–937
diZerega GS, Campeau JD (2001) Peritoneal repair and post-surgical adhesion formation. Hum Reprod Updat 7(6):547–555
diZerega GS (2000) Morphogenesis of adhesion formation. In: DiZerega GS (ed) Peritoneal surgery. Springer, New York, pp 17–23
Forestier D, Slim K, Joubert-Zakeyh J, Nini E, Déchelotte P, Chipponi J (2002) Do bipolar scissors increase postoperative adhesions? An experimental double-blind randomized trial. Ann Chir 127(9):680–684
Mecke H, Riedel HH, Möhlmann L, Lehmann-Willenbrock E, Becker K, Mees C (1988) An animal experiment study. Zentralbl Gynakol 110(7):433–442
Canis M, Matsuzaki S, Bourdel N, Jardon K, Cotte B, Botchorishvili R, Rabischong B, Mage G (2007) Peritoneum and laparoscopic environment. Bull Cancer 94(12):1043–1051
Lundorff P, Hahlin M, Källfelt B, Thorburn J, Lindblom B (1991) Adhesion formation after laparoscopic surgery in tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril 55(5):911–915
Maier DB, Nulsen JC, Klock A, Luciano AA (1992) Laser laparoscopy versus laparotomy in lysis of pelvic adhesions. J Reprod Med 37(12):965–968
Luciano AA, Maier DB, Koch EI, Nulsen JC, Whitman GF (1989) A comparative study of postoperative adhesions following laser surgery by laparoscopy versus laparotomy in the rabbit model. Obstet Gynecol 74(2):220–224
Fowler JM, Hartenbach EM, Reynolds HT, Borner J, Carter JR, Carlson JW, Twiggs LB, Carson LF (1994) Pelvic adhesion formation after pelvic lymphadenectomy: comparison between transperitoneal laparoscopy and extraperitoneal laparotomy in a porcine model. Gynecol Oncol 55(1):25–28
Elkelani OA, Molinas CR, Mynbaev O, Koninckx PR (2002) Prevention of adhesions with cristalloids during laparoscopic surgery in mice. J Am Assoc Gynecol Laparosc 9(4):447–452
Molinas CR, Mynbaev O, Pauwels A, Novak P, Koninckx PR (2001) Peritoneal mesothelial hypoxia during pneumoperitoneum is a cofactor in adhesion formation in a laparoscopic mouse model. Fertil Steril 76(3):560–567
diZerega GS, Tulandi T (2008) Prevention of intra-abdominal adhesions in gynaecological surgery. Reprod Biomed Online 17(3):303–306
Anegg U, Rychlik R, Smolle-Jüttner F (2008) Do the benefits of shorter hospital stay associated with the use of fleece-bound sealing outweigh the cost of the materials? Interact Cardiovasc Thorac Surg 7(2):292–296 discussion 226
Frena A, Martin F (2006) How to improve bilio-stasis in liver surgery. Chir Ital 58(6):793–795
Tagliabue F, D'Angelo C, Zuccon W, Giorgetta C, Gambarini F, Bonandrini L (2007) Use of Tachosil in splenectomy in patients with clotting and blood composition disorders. Minerva Chir 62(1):73–78
Biserte J (2007) TachoSil: the value of its use in urologic surgery. An interview with Professor Jacques Bisertes. J Chir (Paris) 144(1):82–83
Siemer S, Lahme S, Altziebler S, Machtens S, Strohmaier W, Wechsel HW, Goebell P, Schmeller N, Oberneder R, Stolzenburg JU, Becker H, Lüftenegger W, Tetens V, Van Poppel H (2007) Efficacy and safety of TachoSil as haemostatic treatment versus standard suturing in kidney tumour resection: a randomised prospective study. Eur Urol 52(4):1156–1163
Sagalowsky AI (2007) Editorial comment on: efficacy and safety of TachoSil as haemostatic treatment versus standard suturing in kidney tumour resection: a randomised prospective study. Eur Urol 52(4):1162–1163
Abu Hilal M, Hallam MJ, Zeidan BA, Pearce NW (2007) Management of a ruptured pseudoaneurysm of common hepatic artery following pancreaticduodenectomy. ScientificWorldJournal 7:1658–1662
Erdogru T, Sanli A, Celik O, Baykara M (2008) Laparoscopic transvesical repair of recurrent vesicovaginal fistula using with fleece-bound sealing system. Arch Gynecol Obstet 277(5):461–464
Nordentoft T, Rømer J, Sørensen M (2007) Sealing of gastrointestinal anastomoses with a fibrin glue-coated collagen patch: a safety study. J Invest Surg 20(6):363–369
Onorati F, Pasceri E, Scalas C, Santarpino G, Mastroroberto P, Indolfi C, Renzulli A (2008) Aortic tube grafts wrapping with hemostatic fleeces reduces postoperative pericardial effusions. J Cardiovasc Surg (Torino) 49(3):393–397
Tamasauskas A, Sinkūnas K, Draf W, Deltuva V, Matukevicius A, Rastenyte D, Vaitkus S (2008) Management of cerebrospinal fluid leak after surgical removal of pituitary adenomas. Medicina (Kaunas) 44(4):302–307
Attar KH, Namasivayam J, Green J, Peters J (2008) Kidney salvage using the fibrinogen and thrombin-coated sponge TachoSil during nephron-sparing surgery for the resection of large renal tumours. Ann R Coll Surg Engl 90(5):8–11
Lacanna F, Brunati A, Reding R (2008) A new biological mesh for cut surface hemostasis in liver transplantation using technical variants. Pediatr Transplant 12(5):520–521
Shimamoto T, Marui A, Nishina T, Saji Y, Komeda (2008) The TachoSil-Pledget stitch: towards eradication of suture hole bleeding. Ann Thorac Surg 86(6):2002–2004
Getman V, Devyatko E, Wolner E, Aharinejad S, Mueller MR (2006) Fleece bound sealing prevents pleural adhesion. Interact Cardiovasc Thorac Surg 5(3):243–246
Agdi M, Tulandi T (2008) Endoscopic management of uterine fibroids. Best Pract Res Clin Obstet Gynaecol 22(4):707–716
Fedele L, Bianchi S, Zanconato G, Bergamini V, Berlanda N (2004) Bipolar electrocoagulation versus suture of solitary ovary after laparoscopic excision of ovarian endometriomas. J Am Assoc Gynecol Laparosc 11(3):344–347
Li CZ, Liu B, Wen ZQ, Sun Q (2008) The impact of electrocoagulation on ovarian reserve after laparoscopic excision of ovarian cysts: a prospective clinical study of 191 patients. Fertil Steril (in press)
Conflict of interest
There is no actual or potential conflict of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nohuz, E., Darcha, C., Moreno, W. et al. Efficiency of TachoSil® to prevent postsurgical adhesion development on laparoscopic rat model. Gynecol Surg 6, 323–329 (2009). https://doi.org/10.1007/s10397-009-0496-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10397-009-0496-0