Abstract
Background
The aim of this study was to assess the relative prognostic value of biomarkers to measure the systemic inflammatory response (SIR) and potentially improve prognostic modeling in patients undergoing potentially curative surgery for esophageal adenocarcinoma (EC).
Methods
Consecutive 330 patients undergoing surgery for EC between 2004 and 2018 within a regional UK cancer network were identified. Serum measurements of haemoglobin, C-reactive protein, albumin, modified Glasgow Prognostic Score (mGPS), and differential neutrophil to lymphocyte ratio (NLR) were obtained before surgery, and correlated with histopathological factors and outcomes. Primary outcome measures were disease-free (DFS) and overall survival (OS).
Results
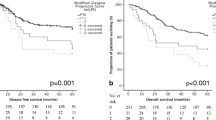
Of 330 OC patients, 294 underwent potentially curative esophagectomy. Univariable DFS analysis revealed pT, pN, pTNM stage (all p < 0.001), poor differentiation (p = 0.001), vascular invasion (p < 0.001), R1 status (p < 0.001), perioperative chemotherapy (p = 0.009), CRP (p = 0.010), mGPS (p = 0.011), and NLR (p < 0.001), were all associated with poor survival. Multivariable Cox regression analysis of DFS revealed only NLR [Hazard Ratio (HR) 3.63, 95% Confidence Interval (CI) 2.11–6.24, p < 0.001] retained significance. Multivariable Cox regression analysis of OS revealed similar findings: NLR [HR 2.66, (95% CI 1.58–4.50), p < 0.001].
Conclusion
NLR is an important SIR prognostic biomarker associated with DFS and OS in EC.
Similar content being viewed by others
Introduction
Biomarkers, at their best, deliver data in three important domains. First, to help diagnose conditions (identifying early stage cancers—diagnostic); second, to forecast aggressive conditions (prognostic); and third, to predict how well a patient will respond to treatment (predictive) [1].
Esophageal cancer (EC) is the sixth leading worldwide cause of cancer related death, accounting for some half a million deaths annually [2]. Surgery remains the only potentially curative treatment, yet almost half of patients develop recurrence, and adjuvant therapies, including chemotherapy or chemoradiotherapy lack global consensus, with no established standard of care [3]. Cancer-related inflammation has been dubbed the 7th hallmark of cancer [4], and the systemic inflammatory response (SIR) is measured using cellular (whole white cell counts, neutrophils, lymphocytes, and platelets), and humoral (C-reactive protein (CRP) and albumin) components. Derivative biomarkers neutrophil–lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), neutrophil-platelet score (NPS), and the modified Glasgow Prognostic Score (mGPS), have also been reported to be associated with poor survival [5,6,7]. If SIR is to become a therapeutic target then a single, sensitive, specific, and reproducible marker is essential, but so far, although multivariable regression models have incorporated common clinic-pathological factors, no study has scrutinized the relative prognostic significance of SIR derived biomarkers in EC.
The aim of this study was to determine if a single biomarker of SIR was independently associated with survival, after potentially curative esophagectomy for cancer. The hypothesis was that a composite biomarker of SIR would have independent significant prognostic value, regardless of histopathological TNM stage, and other SIR biomarkers on multivariable regression modeling. The setting was a regional UK cancer network serving a population of 1.8 million.
Methods
Patients
In order to test the hypothesis a single cohort was developed including patients of radiological TNM stage I to III, deemed to have potentially curable esophageal adenocarcinoma, between January 2004 and August 2018, and treated by a cancer network specialist multidisciplinary team, serving a population of 1.8 million. All patients had management plans individually tailored according to factors related to both patient and disease. Staging was by means of computed tomography, endoscopic ultrasound, computed tomography positron emission tomography, and staging laparoscopy as appropriate [8]. The network MDT treatment algorithms for EC have been described previously [9].
The standard operative approach of subtotal Trans Thoracic esophagectomy (TTO) as described by Lewis [10] and Trans Hiatal esophagectomy (THO), as described by Orringer [11], was used selectively in patients with adenocarcinoma of the lower third of the oesophagus who had significant cardiorespiratory co-morbidity, cT1/2 cN0 or cT3 cN0 disease. A modified extended D2 lymphadenectomy (preserving pancreas and spleen where possible) was performed in all cases. Sixteen patients underwent laparoscopic assisted surgery during the study period. All patients received an Enhanced Recovery After Surgery (ERAS) programme as described previously [12, 13]. Fit patients with tumours of stage cT3 or equivocal cT4 and cN0 or any cT and cN1 were treated with neoadjuvant therapy before surgery [13]. The majority of these patients received 2 cycles of 80 mg/m2 of Cisplatin and 1000 mg/m2 of 5-Fu for 4 days. A minority received 4 cycles of Epirubicin (50 mg/m2), Cisplatin (60 mg/m2) and 5-Fu (200 mg/m2) or Capecitabine (625 mg/m2; ECF/X).
Ethical approval was sought, but the chair of Cardiff & Value University Health Board ethics committee confirmed that individual patient consent was not required to report clinical outcomes alone, and no formal approval was necessary.
Clinicopathological characteristics
Tumours were staged using the seventh edition of the AJCC/UICC-TNM staging system. Pathological factors were recorded from reports issued at the time of surgery and included tumour differentiation, vascular invasion, margin status, and the number of lymph nodes with and without metastasis.
Laboratory whole white-cell count, neutrophil count, lymphocyte count, platelet counts, CRP, and albumin prior to surgery were recorded as described by others [14, 15]. Derivate measurements of the SIR consisted of NLR, NPS, PLR and the mGPS were calculated. These derivative measurements were dichotomised into low and high groups by 2.5 for NLR, and 150 for PLR [6]. The NPS was constructed by grouping patients into three cohorts; zero (0) for patients with both normal neutrophil (≤ 7.5 × 109/L) and platelet counts (≤ 400 × 109/L), one (1) for patients with either a high neutrophil (> 7.5 × 109/L) or platelet count (> 400 × 109/L), and two (2) for patients with both high neutrophil and platelet count. The mGPS was constructed using CRP and albumin. Patients with normal serum levels of CRP (≤ 10 mg/l) and albumin (≥ 35 g/l) were given a score of zero. Patients with a raised serum CRP (> 10 mg/l) and normal serum albumin were given a score of one, and patients with a raised serum CRP and low serum albumin (< 35 g/l) were given a score of two [16].
Patients were followed up at regular intervals of 3 months for the first year and 6 months thereafter. In the event that patients developed symptoms suggestive of recurrent disease, investigations were undertaken sooner. The follow-up surveillance was conducted for 5 years or until death. Death certification was obtained from the Office for National Statistics via Cancer Network Information System Cymru (CaNISC).
Statistical analysis
Sample size calculations were based on a pre-study literature survey of (CRUK cancers statistics [17]), which indicated that the baseline five-year survival rate of patients diagnosed with stage II EC was expected to be 40%, compared with 20% in patients with stage III EC, and a 15% difference in survival would be a realistic expectation. Thus, a minimum of 276 patients were to be studied, providing 80% power to detect such a difference with alpha set at p < 0.05.
Grouped data were expressed as median (range) and non-parametric methods used throughout. Disease-free survival for all patients was calculated by measuring the interval from a landmark time of 6 months after diagnosis to the date of recurrence. This approach was adopted in previous randomized trials [18], to allow for the variable interval to surgery following diagnosis, depending on whether neoadjuvant therapy was prescribed. As in these trials, events resulting in a failure to complete curative treatment, such as not proceeding to surgery, open and close laparotomy, palliative resection, in-hospital mortality and disease progression during neoadjuvant chemotherapy, were assumed to have occurred at this landmark time, to maintain the intention-to-treat analysis. Overall survival was measured from the date of diagnosis. Cumulative survival was calculated according to the Kaplan–Meier method; differences between groups were analyzed with the log rank test. Univariable analyses examining factors influencing survival were examined initially by the life table Kaplan–Meier method, and those with associations found to be significant on log-rank analysis (p < 0·100) were retained in a Cox proportional hazards model using forward conditional methodology to assess the prognostic value of individual variables. All statistical analysis was performed in SPSS® (IBM® SPSS® Statistics v25.0.0.0, IBM Corporation, Armonk, New York, USA) with extension R.
Results
Patients, clinico-pathological factors and features associated with non-resectability
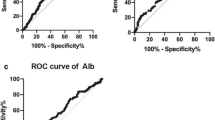
In total, 330 patients were identified who underwent surgery for EC. Thirty-six patients (10.9%) were deemed to have inoperable tumours because of local invasion. The remaining 294 patients underwent potentially curative esophagectomy. The patient cohort undergoing palliative surgery had raised serum CRP measurements (41.7% vs. 16.0%, p < 0.001), hypoalbuminaemia (36.1% vs. 16.7%, p = 0.005), higher mGPS (27.8% vs. 6.6%, p < 0.001), thrombophilia (13.9% vs. 2.7%, p = 0.001), higher NPS (2.8% vs 0.3%, p = 0.006), and were more likely to have received neoadjuvant chemotherapy (91.4% vs. 70.4%, p = 0.008). On multivariable binary logistical regression analysis of factors associated with poor survival on univariable analysis, mGPS (Odds Ratio (OR) 2.29 (95% Confidence Interval (95% CI) 1.44–3.62), p < 0.001), thrombophilia (OR 4.76 (1.26–18.06), p = 0.022) and neoadjuvant therapy (OR 3.72 (1.10–11.57), p = 0.023) were independently associated with inoperability. The area under the curve (AUC) for neoadjuvant therapy was 0.59 (95% CI 0.50–0.68, p = 0.079), AUC for thrombophilia 0.59 (95% CI 0.45–0.67, p = 0.263), and AUC for mGPS 0.64 (95% CI 0.54–0.75, p = 0.006).
Details of the patients undergoing potentially curative esophagectomy
The characteristics of clinico-pathological variables studied can be found in Tables 1 and 2. The median age for patients undergoing resection was 69 years (inter-quartile range (IQR) 62–74) with the majority (n = 141, 48.0%) being between 65 and 75 years of age (Table 2). Most patients were male (n = 250, 85.0%), and were lymph node positive (n = 149, 50.7%). Perioperative chemotherapy was prescribed in 207 patients (70.4%, Tables 1 and 2). During follow-up, 86 patients (29.2%) developed cancer recurrence and 106 patients (36.1%) died. Median follow-up of the surviving patients was 34 (range 6–60) months and 194 (58.8%) of patients were followed-up for 5 years or until death.
Relationships between the SIR, clinico-pathological factors and survival
Univariable and multivariable analyses of the factors associated with both disease-free and overall survival can be found in Table 3. The number of events per variable was 10.6. The relationship between NLR and clinico-pathological factors is shown in Table 4.
Discussion
The principal finding of this study was that NLR emerged as the only significant inflammatory prognostic biomarker, in a cohort of UK patients with EC, supporting the primary hypothesis. No fewer than one in six patients had raised SIR markers, and some one in ten a raised NLR. Patients with mGPS of two were nearly five times more likely to have inoperable cancers when compared with patients with mGPS of zero. Moreover, elevated NLR, CRP, and mGPS, were all associated with poorer disease-free and overall survival profiles. Patients with a low NLR experienced median DFS and OS, on average 18 and 13 months respectively better than patients with a high NLR. Similarly, patients with a low NLR experienced five-year DFS and OS of 45.3%, and 49.6%, 1.6 and 1.8 fold better than patients with a high NLR.
The inflammatory markers described in this study can be broadly characterized as hepatic (mGPS and its components) or haematological (NLR, NPS, PLR, and their components), based on their predominant area of activity. NLR and mGPS have been associated with poor survival in a raft of anatomical cancer sites including breast [19, 20], colorectal [21, 22], stomach [6, 23], and prostate [24, 25]. In gastric [6], colorectal [22], and prostate [24] cancer patients with a mGPS of two had a five-year survival of 20%, 45%, and 33%, compared with 80%, 85%, and 75% in paients with mGPS of zero respectively. With regard to mGPS and NLR, 16.5% and 10.6% of patients respectively, had evidence of SIR on pre-operative blood analysis. This compares with previous reports citing SIR of 20% (mGPS), and 10% (NLR) of gastric cancer patients [6], and 41% [26] (mGPS) and 19% (NLR) of colorectal cancer patients [5]. But in contrast to reports in gastric and colorectal cancer, NLR was the only prognostic inflammatory biomarker found to be associated with survival in EC. Cumulative 5-year differential survival related to NLR expression have been reported to be similar in both esophageal (17%) and colorectal cancer (20%) [5]. Yet the absolute 5-year survival in the patient cohorts with low NLR expression was 45% in esophageal, compared with 75% in colorectal cancer [5]; arguably consistent with the more aggressive clinical nature of EC when compared with colorectal cancer.
The underlying basis of the relationship between the SIR and poor cancer survival in patients with EC is an enigma. But the components of the mGPS have been characterized, with cancer cachexia, compromised cellular immune response, angiogenesis, and the up-regulation of growth factors all prominent features. It is evident therefore that the mechanisms controlling the association between systemic inflammation and cancer-specific survival are compound. Immune dysfunction, growth and dissemination, nutritional and functional decline, and tumour angiogenesis, are all implicated. More explicitly, recent colorectal cancer research has indicated a strong relationship between suboptimal patient physiological stage, increased comorbidity, and elevated CRP. In patients with an Eastern Cooperative Oncology Group Performance Status (ECOG-PS) zero or one, the median CRP was 20 mg/dl, compared with 64 mg/dl in the ECOG-PS of two (p = 0.002). Arguably, a raised preoperative mGPS in EC resonates with poor cardiorespiratory fitness, more comorbidity, and higher risk profile [27]. Most nutrition research professionals presently hold the view that in patients with cancer, nutritional status is closely related to the presence of a systemic inflammatory response as supported by the GPS, NLR, PLR, and NPS [28]. Indeed, cachexia is now considered by a number of expert nutrition groups to constitute disease related malnutrition in addition to inflammation, and comprises an etiologic factor in the GlIM criteria [29]. Future research should not only assess nutritional status but also the systemic inflammatory status in patients with EC cancer. The adverse prognostic power of the SIR is likely multifactorial in nature representing a more aggressive cancer phenotype on a background of a suppressed host response. However, this would be difficult to prove using association studies alone. Nevertheless, the data here confirms that survival following cancer is largely determines by the TNM stage, vascular invasion, and the presence of a SIR (Fig. 1).
Although SIR biomarkers offer valuable prognostic signals, if NLR and mGPS are to be incorporated into an upgraded TNM staging system, they should add prognostic value related to treatment response. Inclusion of biomarkers into gastric and breast cancer management algorithms was driven by the identification of adjuvant therapies for higher risk patients. Apart from Herceptin treatment for advanced esophago-gastric cancer, the principal adjuvant treatment for EC remains cytotoxic chemotherapy, despite pathological response rates of the order of one in seven [30]. Reports regarding the treatment of colorectal cancer with neoadjuvant [31, 32] or adjuvant chemotherapy [33] describe poorer outcomes in patients with SIR when compared with controls. Moreover, based on histological assessment, mGPS in rectal cancer has been reported to be associated with significantly poorer response to neoadjuvant chemotherapy [32]. Powell et al. from Cardiff, have also reported that an elevated pre-treatment NLR was associated with poorer tumour regression grade in patients undergoing neoadjuvant chemotherapy for EC [34]. Given the associations between the SIR and relative chemo-resistance, it is unlikely that such patients will derive any discernable clinical benefit from adjuvant chemotherapy. The phase III, double blinded, placebo controlled randomized trial regarding the effect of aspirin on disease recurrence and survival after primary therapy in non-metastatic solid tumours (Add-Aspirin), commenced recruitment in 2015, and should signal whether Aspirin is beneficial in EC treatment; yet arguably only patients with a SIR will respond. In keeping with other adjuvant treatments such as Herceptin and Cetuximab, patient selection is crucial and NLR has predictive biomarker potential in this regard.
There are a number of potential inherent limitations to studies of this character, which have been described previously [6, 35]. Cohort sample size was modest and study power was built on a 15% survival difference; sub-analysis related to patient comorbid risk profile, tumour stage, and morbidity severity-score was therefore not practical, and the above are therefore clearly confounding factors. Validating the results in an appropriately powered cohort for sub-analysis stage-for-stage, may facilitate the integration of NLR into a modified TNM staging system [36]. Though several SIR related bio-markers were used, the NLR may not predict survival independently in other patient cohorts and this matter needs international authentication. In contrast, the study has strengths. Patients were recruited from a consecutive patient cohort diagnosed with EC, from a single UK geographical region, all treated by a specialist multi-disciplinary team with standardized stage tailored treatment algorithms, operative techniques, with international peer-reviewed and published key performance indicator quality control. The survival data is particularly strong; no patients were lost to follow-up, and causes and dates of death were obtained from the office of national statistics. Moreover, the study builds on previous research by including all clinically available inflammatory and pathological factors in a multivariable regression model, and adds to the established evidence base, regarding the prognostic significance of preoperative SIR.
In summary, NLR was the sole inflammatory based prognostic biomarker associated with poor DFS and OS after potentially curative esophagectomy for cancer and was independent of histopathological stage. These findings emphasise the importance of not only staging the patient’s tumour radiologically; in essence a perceived histopathological stage; but also staging the patient’s physiological state using derivative SIR biomarkers. The results of this study consolidate the prognostic value of combined markers of the SIR, with the mGPS predicting patients at risk of inoperable disease at the time of surgery. This simple blood profile work deserves further considered reflection and should form part of routine preoperative patient work-up, and follow-up, for all such patients undergoing resection for cancer. These findings suggest that SIR presents narrative remedial goals, for patients vulnerable to cancer recurrence, and NLR may be the best biomarker to guide tailored holistic anti-inflammatory therapy, but further mechanistic studies are needed.
Data availability
We will not be making the dataset available, however, are more than happy to answer any questions of offer further information as required.
Change history
12 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10388-021-00836-y
References
Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005;5:845–56.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin Am Cancer Soc. 2018;68:394–424.
du Rieu MC, Filleron T, Beluchon B, Humeau M, Julio C-H, Bloom E, et al. Recurrence risk after Ivor Lewis oesophagectomy for cancer. J Cardiothorac Surg BioMed Central. 2013;8:215.
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–81.
Dolan RD, McSorley ST, Park JH, Watt DG, Roxburgh CS, Horgan PG, et al. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: comparison of composite ratios and cumulative scores. Br J Cancer. 2018;119:40–51.
Powell AGMT, Parkinson D, Patel N, Chan D, Christian A, Lewis WG. Prognostic significance of serum inflammatory markers in gastric cancer. J Gastrointest Surg. 2018;22:595–605.
Ishibashi Y, Tsujimoto H, Yaguchi Y, Kishi Y, Ueno H. Prognostic significance of systemic inflammatory markers in esophageal cancer: systematic review and meta-analysis. Ann Gastroenterol Surg. 2020;4:56.
Patel N, Foley KG, Powell AG, Wheat JR, Chan D, Fielding P, et al. Propensity score analysis of 18-FDG PET/CT-enhanced staging in patients undergoing surgery for esophageal cancer. Eur J Nucl Med Mol Imaging. 2019;46:801–9.
Morgan MA, Lewis WG, Casbard A, Roberts SA, Adams R, Clark GWB, et al. Stage-for-stage comparison of definitive chemoradiotherapy, surgery alone and neoadjuvant chemotherapy for oesophageal carcinoma. Br J Surg. 2009;96:1300–7 (John Wiley & Sons).
Lewis I. The surgical treatment of carcinoma of the oesophagus with special reference to a new operation for growths of the middle third. Br J Surg. 1946;34:18–311.
Orringer MB. Transhiatal esophagectomy for benign disease. J Thorac Cardiovasc Surg. 1985;90:649–55.
Karran A, Wheat J, Chan D, Blake P, Barlow R, Lewis WG. Propensity score analysis of an enhanced recovery programme in upper gastrointestinal cancer surgery. World J Surg. 2016;40:1645–54.
Patel N, Powell AG, Wheat JR, Brown C, Appadurai IR, Davies RG, et al. Cardiopulmonary fitness predicts postoperative major morbidity after esophagectomy for patients with cancer. Physiol Rep. 2019;7:e14174 (Wiley).
Dutta S, Crumley ABC, Fullarton GM, Horgan PG, McMillan DC. Comparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative resection of oesophageal cancer. World J Surg. 2011;35:1861–6 (Springer-Verlag).
Walsh SM, Casey S, Kennedy R, Ravi N, Reynolds JV. Does the modified Glasgow Prognostic Score (mGPS) have a prognostic role in esophageal cancer? J Surg Oncol. 2016;113:732–7.
Dutta S, Crumley ABC, Fullarton GM, Horgan PG, McMillan DC. Comparison of the prognostic value of tumour and patient related factors in patients undergoing potentially curative resection of gastric cancer. Am J Surg Elsevier. 2012;204:294–9.
Cancer Research UK. Oesophageal cancer survival statistics [Internet]. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/oesophageal-cancer/survival. Accessed 28 Aug 2020
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJH, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20 (Massachusetts Medical Society).
Al Murri AM, Bartlett JMS, Canney PA, Doughty JC, Wilson C, McMillan DC. Evaluation of an inflammation-based prognostic score (GPS) in patients with metastatic breast cancer. Br J Cancer. 2006;94:227–30 (Nature Publishing Group).
Ethier J-L, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19:2.
Shibutani M, Maeda K, Nagahara H, Noda E, Ohtani H, Nishiguchi Y, et al. A high preoperative neutrophil-to-lymphocyte ratio is associated with poor survival in patients with colorectal cancer. Anticancer Res. 2013;33:3291–4 (International Institute of Anticancer Research).
Park JH, Powell AG, Roxburgh CSD, Horgan PG, McMillan DC, Edwards J. Mismatch repair status in patients with primary operable colorectal cancer: associations with the local and systemic tumour environment. Br J Cancer. 2016;114:562–70.
Mellor KL, Powell AGMT, Lewis WG. Systematic review and meta-analysis of the prognostic significance of neutrophil-lymphocyte ratio (NLR) after r0 gastrectomy for cancer. J Gastrointest Cancer. 2018;49:237–44.
Shafique K, Proctor MJ, McMillan DC, Leung H, Smith K, Sloan B, et al. The modified Glasgow prognostic score in prostate cancer: results from a retrospective clinical series of 744 patients. BMC Cancer. 2013;13:292.
Gu X, Gao X, Li X, Qi X, Ma M, Qin S, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in prostate cancer: evidence from 16266 patients. Sci Rep. 2016;6:22089 (Nature Publishing Group).
Park JH, Watt DG, Roxburgh CSD, Horgan PG, McMillan DC. Colorectal cancer, systemic inflammation, and outcome. Ann Surg. 2016;263:326–36.
Dolan RD, Laird BJA, Klepstad P, Kaasa S, Horgan PG, Paulsen Ø, et al. An exploratory study examining the relationship between performance status and systemic inflammation frameworks and cytokine profiles in patients with advanced cancer. Medicine (Baltimore). 2019;98:e17019.
Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36:1187–96.
Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition—a consensus report from the global clinical nutrition community. Clin Nutr. 2019;38:1–9.
Noble F, Lloyd MA, Turkington R, Griffiths E, O’Donovan M, O’Neill JR, et al. Multicentre cohort study to define and validate pathological assessment of response to neoadjuvant therapy in oesophagogastric adenocarcinoma. Br J Surg. 2017;104:1816–28.
Carruthers R, Tho LM, Brown J, Kakumanu S, McCartney E, McDonald AC. Systemic inflammatory response is a predictor of outcome in patients undergoing preoperative chemoradiation for locally advanced rectal cancer. Color Dis. 2012;14:e701–e707707.
Dreyer SB, Powell AGMT, McSorley ST, Waterston A, Going JJ, Edwards J, et al. The pretreatment systemic inflammatory response is an important determinant of poor pathologic response for patients undergoing neoadjuvant therapy for rectal cancer. Ann Surg Oncol. 2017;24:1295–303.
Ishizuka M, Nagata H, Takagi K, Kubota K. Influence of inflammation-based prognostic score on mortality of patients undergoing chemotherapy for far advanced or recurrent unresectable colorectal cancer. Ann Surg. 2009;250:268–72.
Powell AGMT, Chin C, Coxon AH, Chalishazar A, Christian A, Roberts SA, et al. Neutrophil to lymphocyte ratio as a predictor of response to neoadjuvant chemotherapy and survival in oesophageal adenocarcinoma. BJS Open. 2020;1:1 (John Wiley & Sons Ltd).
Powell A, Coxon AH, Patel N, Chan D, Christian A, Lewis W. Prognostic significance of post-operative morbidity severity score after potentially curative D2 gastrectomy for carcinoma. J Gastrointest Surg. 2018;22:1516–27.
Chan DSY, Reid TD, White C, Willicombe A, Blackshaw G, Clark GW, et al. Influence of a regional centralised upper gastrointestinal cancer service model on patient safety, quality of care and survival. Clin Oncol. 2013;25:719–25.
Acknowledgements
The authors would like to acknowledge the assistance of Professor Donald McMillan, of The University of Glasgow, for his advice in preparing the manuscript. The members of South East Wales Oesophagogastric Cancer Collaboratives are: Guy Blackshaw, Anuja Chalishazar, Geoffrey Clark, Xavier Escofet, Antonio Foliaki, Timothy Havard, Mark Henwood, Osian James, Neil Patel, Jolene Witherspoon, Wyn Lewis.
Funding
Not applicable.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by AP, CE, CC, AC, AC, and WGL. The first draft of the manuscript was written by AP and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
Details around ethical approval can be found in the methodology section. This states that “Ethical approval was sought, but the chair of Cardiff & Value University Health Board ethics committee confirmed that individual patient consent was not required to report clinical outcomes alone, and no formal approval was necessary.”
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of South East Wales Oesophagogastric Cancer Collaborative are listed in acknowledgements.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Powell, A.G.M.T., Eley, C., Chin, C. et al. Prognostic significance of serum inflammatory markers in esophageal cancer. Esophagus 18, 267–277 (2021). https://doi.org/10.1007/s10388-020-00772-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-020-00772-3